Key points:
- Interventional cardiology and percutaneous coronary intervention has evolved from balloon angioplasty and bare metal stents (BMSs) towards contemporary drug-eluting stent (DES) devices and novel biodegradeable, bioresorbable scaffolds and stents.
- First generation DESs significantly reduced risk of in-stent restenosis observed with BMSs but their use was complicated by late and very late stent thrombosis.
- Second generation DESs dramatically decreased this risk and rate of one-year stent thrombosis is currently under 1%.
- Bioresorbable scaffolds, biodegradable DESs and polymer-free, drug-coated stents are associated with low clinical event rates.
- Special considerations are warranted in high-risk patients when using stent devices.
Introduction
Percutaneous coronary intervention (PCI) is the standard of care for patients with symptomatic coronary artery disease on optimal medical therapy[1]
and each year more than 300,000 patients undergo coronary stenting in the United States[2]
. The aim of PCI is to alleviate symptom frequency and reduce morbidity associated with repeated hospitalisation. Invasive revascularisation is the definitive treatment option for ST segment elevation myocardial infarction (STEMI) where facilities for primary PCI are available and when patients present within guideline recommended times[3],[4]
. Stent implantation is supported with dual antiplatelet therapy (DAPT) to ensure vessel patency (degree of openness)[5]
.
PCI originated more than two decades earlier and primarily consisted of balloon angioplasty. It rapidly evolved to bare metal stent (BMS) implantation, which overcame the limitations of angioplasty related to vessel recoil and binary restenosis (≥50% luminal narrowing at follow-up angiography)[6]
. Continued advances led to the development of first generation drug-eluting stents (DESs), which incorporated an antiproliferative drug in a polymer matrix, enveloping the metal stent. These first generation DESs significantly decreased the risk of restenosis and need for repeat revascularisation associated with BMSs[7],[8]
. Despite remarkable initial success, DES use was complicated by late and very late stent thrombosis (ST) caused by polymer hypersensitivity and delayed endothelialisation[9],[10],[11],[12]
. However, improvements in stent type and polymer biocompatibility facilitated development of second generation DESs, which have consistently shown very low ST rates[13],[14],[15],[16]
.
More recent stent technologies include bioresorbable devices, bioabsorbable polymer stents and endothelial progenitor cell technology stents[17],[18],[19]
. Bioresorbable devices include an absorbable scaffold either polymeric or metal with an antiproliferative drug[20],[21]
. Biodegradable polymer stents have a resorbable polymer and antiproliferative drug matrix embedded in a metal stent and the polymer is completely absorbed between four and nine months[18],[22]
. Endothelial progenitor cell technology stents include luminally coated anti-CD34 antibodies that combine with circulating endothelial progenitor cells and the abluminally coated antiproliferative drug sirolimus[19]
. These stent types aim to promote healthy endothelialisation and shorter periods of DAPT for decreasing ST risk.
Concurrently, choice of antithrombotic therapy and duration of DAPT impact on the net benefit of PCI. Consequently, tailored patient management for stent choice and pharmacotherapy is crucial. This review will discuss the evolution of DESs and outcomes from clinical trials, as well as performance of DESs in specific populations.
Sources and selection criteria
To identify suitable scientific studies for this review, a comprehensive search was performed on PubMed using combinations of the search terms ‘percutaneous coronary intervention’, ‘bare metal stents’ and ‘drug eluting stents’, ‘randomized controlled trial’ and ‘observational registry’ from 2000 to 2016 inclusive. Other articles, including societal guidelines and conference reports, were identified via Google search.
Mechanism of action
The two main antiproliferative agents used in DESs are rapamycins (limus drugs – sirolimus and its analogues) and taxanes (paclitaxel).
First generation DESs include sirolimus-eluting stents (SESs) and paclitaxel-eluting stents (PESs). These stents were superseded by second generation DESs, namely, everolimus-eluting stents (EESs) and zotarolimus-eluting stents (ZESs), and third generation DESs, namely, biolimus-eluting stents (BESs) and novolimus eluting stents (NESs).
The limus family of drugs acts on the mammalian TOR receptor (m-TOR), blocking cell cycle between the G1 and S phases to inhibit smooth muscle cell proliferation[23]
. Sirolimus is a cytostatic drug (it suppresses cell growth) and the mechanism of action of all other limus drugs (everolimus, zotarolimus, biolimus, novolimus) is similar to sirolimus. Novolimus is a metabolite of sirolimus that requires a lower concentration of both drug and polymer to achieve effective inhibition of neointimal hyperplasia[24]
.
Paclitaxel, on the other hand, has a different mechanism of action to the limus drugs. It is an inhibitor of microtubule depolymerisation and causes cell cycle arrest in the G0/1 and G2/M phases[23]
.
Stent and polymer materials
Table 1 shows the different types of DESs with details on stent and polymer material. Figure 1 illustrates the evolution of stent technologies.
| Table 1: Stent types and materials | |||
|---|---|---|---|
| Stent name, Manufacturer | Scaffold material | Antiproliferative agent | Polymer type |
| Permanent polymer permanent scaffold | |||
| First generation DESs | |||
| Sirolimus eluting stent, Cypher, Cordis | Stainless steel | Rapamycin or sirolimus | Durable, polyethylene-co-vinyl acetate (PEVA) and poly n-butyl methacrylate (PBMA) |
| Paclitaxel eluting stent, Taxus, Boston Scientific | Taxus Express and Taxus Liberte – stainless steel; Taxus Element – Platinum chromium | Paclitaxel | Durable, polystyrene-b-isobutylene-b-styrene |
| Second generation DESs | |||
| Everolimus eluting stent, Xience, Abbott Vascular | Cobalt chromium | Everolimus | Durable, PBMA, a polymer that adheres to the stent and drug coating, and semi-crystalline random copolymer PVDF-HFP, which is composed of vinylidene fluoride and hexafluoropropylene monomers |
| Everolimus eluting stent, Promus, Boston Scientific | Platinum chromium | Everolimus | Durable, primer polymer layer, PBMA that functions as an adhesion promoter between the bare metal and the drug matrix layer. Drug matrix contains a semi-crystalline random copolymer, PVDF-HFP, poly (vinylidene fluoride-co- hexafluoropropylene) |
| Zotarolimus eluting stent, Endeavor, Medtronic | Cobalt chromium | Zotarolimus | Phosphorylcholine polymer |
| Zotarolimus eluting stent, Resolute, Medtronic | Cobalt chromium | Zotarolimus | BioLinx copolymer system containing polyvinylpyrrolidone |
| Bioresorbable scaffolds | |||
| ABSORB, Abbott Vascular | Poly-L-lactic acid (PLLA) | Everolimus | Poly D,L lactide polymer |
| DESolve, Elixir Medical | PLLA | Myolimus | Ultrathin polylactide-based polymer |
| DREAMS 2G, Biotronik | Magnesium alloy WE-43 | Sirolimus | Absorbable polymer polylactic-coglycolic acid |
| Fantom, REVA | Tyrosine polymer scaffold | Sirolimus | Polylactide polymer |
| Biodegradable polymer durable stents | |||
| Biomatrix, Biosensors | Cobalt chromium | Biolimus A9 | Biodegradable PLA technology polymer, abluminal coating |
| Nobori, Terumo | Cobalt chromium | Biolimus A9 | Biodegradable PLA technology polymer, abluminal coating |
| Ultimaster, Terumo | Cobalt chromium | Sirolimus | Bioresorbable poly(DL-lactide-co-caprolactone), circumferential coating |
| Orsiro, Biotronik | Cobalt chromium | Sirolimus | Biodegradable PLLA polymer; amorphous silicon carbide coating on the stent, circumferential coating |
| Synergy, Boston scientific | Platinum chromium | Everolimus | Biodegradable PLLA polymer, poly-lactide-co-glycolide, abluminal coating |
| DESyne, Elixir Medical | Cobalt chromium | Novolimus | Ultrathin polylactide-based polymer |
| Yukon, Translumina therapeutics | Microporous Stainless steel stent, top coat of shellac resin | Sirolimus | Biodegradable Polymer Resomer R202S, abluminal coating |
| Polymer free drug coated stents | |||
| Biofreedom, Biosensors | Microporous Stainless steel | Biolimus A9 | – |
| EPC technology stents | |||
| Combo stent, OrbusNeich | Stainless steel | Sirolimus | Biodegradable; endothelial progenitor cell technology |
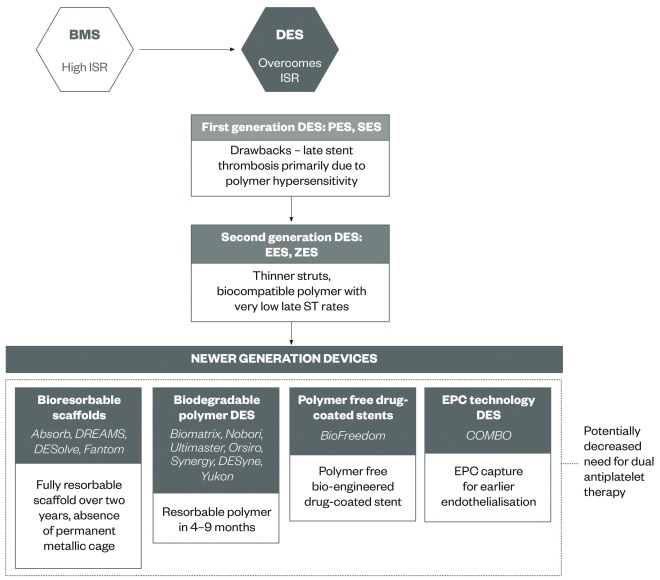
Figure 1: Evolution of coronary stent technology
BMS, bare metal stent; DES, drug-eluting stent; EES, everolimus eluting stent; EPC, endothelial progenitor cell; ISR, in-stent restenosis; PES, paclitaxel eluting stent; SES, sirolimus eluting stent; ZES, zotarolimus eluting stent.
First generation DESs: Both Cypher® SES (Cordis, Florida, United States) and Taxus® PES (Boston Scientific, Massachusetts, United States) are stainless steel stents. While Cypher and Taxus® Express™ have a closed cell design, Taxus® Liberté® has a hybrid design. The Cypher stent has a strut thickness of 140μm, polymer thickness of 12.3μm; 75% of the drug is released over ten days and 80% is released by 30 days[23],[25]
. Taxus Express PES has a strut thickness of 132μm and Taxus Liberté PES has a strut thickness of 97μm with a polymer thickness of 16μm, releasing the drug in a slower manner at <10% over 28 days. On the other hand, Taxus® Element™ is a platinum chromium (Pt-Cr) stent with faster delivery of the drug, which is completely eluted over three months.
Second generation DESs: EES may be composed of a cobalt chromium (CoCr-EES) or PtCr-EES platform. The strut/ polymer thickness is 81/7.8μm and 87% of everolimus is eluted over 90 days[26]
. DESs typically delay endothelialisation, resulting in a prolonged inflammatory state and enhanced platelet aggregation. However, everolimus is associated with more rapid endothelialisation because of faster elution and in animal models has demonstrated complete stent coverage with CD31 antigen within 14 days, a marker of good endothelial function[23]
. ZESs include a biocompatible phosphorylcholine polymer system with a strut/polymer thickness of 91/4.1μm. The BioLinxâ„¢ co-polymer system in the Resolute-ZES (Medtronic Vascular, California, United States) contains polyvinyl pyrrolidone, allowing extended release of zotarolimus – releasing 85% of the drug over 60 days[26]
.
New generation devices: These include bioresorbable scaffolds, biodegradable polymer stents, polymer free stents and endothelial progenitor cell technology stents.
The bioresorbable devices have a polymeric or metal (magnesium) scaffold that is completely resorbed between six months to two years[27]
. The Absorbâ„¢ bioresorbable scaffold (Abbott Vascular, California, United States) has a polymer backbone of poly-L-lactic acid (PLLA) coated with a mixture of poly-D,L lactide polymer and a limus drug[28]
. The DREAMS (drug-eluting absorbable metal scaffold) stent, now the Magmaris stent (Biotronik, Berlin, Germany), incorporates a magnesium alloy metal bioresorbable stent (BRS) with either paclitaxel or sirolimus[29],[30]
.
Biodegradable polymer stents have a resorbable polymer[18]
; whereas some novel stents are polymer-free while incorporating drug elution technology[31],[32]
. Biodegradable polymer BESs have a strut thickness of 112μm and a highly lipophilic 15μm thick polymer, with most of the drug released over one month[18],[33]
. The polymer is completely degraded by Kreb’s cycle over four to nine months[18]
. Other biodegradable polymer stents have thinner struts, such as Orsiro Co-Cr SES (60–80μm) (Biotronik, Berlin, Germany) and SYNERGYâ„¢ Pt-Cr EES (74 micron) (Boston Scientific, Massachusetts, United States)[22]
. With the Orsiro stent, 80% of the drug is released over three months. The SYNERGY stent has an elution profile similar to the Promus Element EES stent (Boston Scientific, Massachusetts, United States) and the polymer is completed resorbed at four months[34]
.
The BioFreedomâ„¢ stent (BioSensors International, Singapore) is an example of a polymer-free drug coated stent[35]
. It has a microporous stainless steel stent with a strut thickness of 119μm coated with Biolimus A9[31]
.
Endothelial progenitor cell technology stents are stainless steel stents with a strut thickness of 100μm, abluminal coating of sirolimus in a biodegradable polymer matrix (3–5μm thick) and luminal coating of antibodies to endothelial progenitor cells[36]
. The polymer is biodegradable over three months and most of the sirolimus is eluted over 35 days.
Clinical data
Several landmark randomised trials have compared DESs against BMSs, and have examined differences in outcomes between first and second generation DESs.
First generation DES trials
The RAndomised study with the sirolimus-eluting bx VELocity balloon-expandable stent (RAVEL) trial was the first DES trial that compared sirolimus coated stents with BMSs in 238 patients across 19 sites. The primary end point was late lumen loss at six months[7]
. At one year, the major adverse coronary event (MACE) rate was 5.8% in the SES group vs. 28.8% in the BMS group. At six months, no patients had ST. The SIRolImUS-eluting stent in de novo coronary artery lesions (a segment of artery-blocking plaque that has not previously been treated with angioplasty or stenting, [SIRIUS]) trial also tested SES against BMS in 1,058 patients and noted the one-year rate of target lesion revascularisation (TLR) to be 4.9% with SES vs. 20.0% with BMS, without differences in death or myocardial infarction (MI)[37]
. A pooled analysis of the SIRIUS, C-SIRIUS and E-SIRIUS studies evaluated 1,510 patients[38]
. The incidence of TLR at two years was significantly lower in SES patients compared with BMS patients (risk ratio [RR] 0.25; 95% confidence interval [CI] 0.18–0.35). Moreover, the incidence of two-year ST was similar (0.9% in SES patients vs. 0.7% in BMS patients).
The TAXUS trials evaluated PESs against BMSs. TAXUS II compared slow release and moderate release PESs with BMSs. At five years, the incidence of MACE was 27.6% in the BMS group vs. 20.4% in the slow release Taxus group vs. 15.1% in the moderate release Taxus group. Similarly, the incidence of TLR was 18.5% vs. 10.3% vs. 4.5% in the three groups, respectively. There were two ST events in the BMS group and no events in the SES groups beyond two years[39]
.
However, first generation DESs resulted in late and very late stent thrombosis, which was considered to be a consequence of polymer hypersensitivity and delayed endothelialisation[9]
,[25]
. Giant cells and eosinophils were noted around polymer fragments, along with induction of apoptosis of smooth muscle cells in cases of late ST, confirming the role of polymer hypersensitivity[40],[41]
. Another important factor contributing to late ST was patient compliance to antiplatelet therapy, which remains relevant despite improved stent technology[42]
. Conversely, early ST may be affected by technical factors, such as ostial or bifurcation lesions, stent underexpansion and stent apposition (lack of contact between stent struts and the underlying vessel wall); as well as patient factors, such as age, hypertension, female gender, renal disease and acute coronary syndrome (ACS)[9]
,[41]
.
Interestingly, recently published ten-year results from the randomised Sirolimus-Eluting vs. Paclitaxel-Eluting Stents for Coronary Revascularisation (SIRTAX) trial showed that although the cumulative ten-year risk of definite ST was similar for SESs and PESs at around 5.6%; the risk of definite ST decreased between five and ten years after PCI to around 1.1%[43]
. The annual risk of very late ST was 0.23% per year between five and ten years, compared with 0.67% per year between one to five years.
Nevertheless, in search of an ideal polymer, several carriers and coatings have been investigated, including hydroxyapatite and heparin[41]
. While second generation DESs include more biocompatible polymers, novel DES types incorporate biodegradable polymers composed of lactic or glycolic acid, which are completely resorbed[18]
or are entirely polymer-free[31]
.
Second generation DES trials
The SPIRIT III trial randomised 1,002 patients to EESs or PESs (2:1). At five years, EESs were associated with lower incidence of death (5.9% vs. 10.1%, P =0.02), MACE (13.2% vs. 20.7%, P =0.007) and target vessel failures (19.3% vs. 24.5%, P =0.05) without differences in MI or ST rates[44]
. SPIRIT IV randomised 3,687 patients undergoing PCI of up to three non-complex de novo lesions to either EESs or PESs. At two years, EESs resulted in lower target lesion failure (6.9% vs. 9.9%, P =0.003) but similar rates of all-cause and cardiac mortality compared with PESs[45]
. For the first time, EESs were also associated with lower ST (0.4% vs. 1.2 %, P =0.008).
The COMPARE randomised controlled trial (RCT) randomised treatment to EESs or PESs (Taxus Liberté stent) in 1,800 all comer patients undergoing PCI. At five years, EESs resulted in lower incidence of the primary composite end point of death, MI, target vessel revascularisation (TVR) (18.4% vs. 25.1%, P =0.0005) compared with PESs. Similarly, the rate of MI (7.0% vs 11.5%, P =0.001) and ST (3.1% vs 5.9%, P =0.005) was lower but mortality was similar (9.0% vs 10.3%, P =0.36)[46]
.
The ENDEAVOR series of trials compared ZESs with BMSs, SESs and PESs, respectively. Compared with BMSs in the Endeavor II trial, ZESs were associated with lower target vessel failure at five years (15.4% vs. 24.4%, P <0.001) but there was no difference in ST (0.9% vs. 1.5%, P =0.42) or death[47]
. In the Endeavor III trial, ZESs compared with SESs resulted in lower five-year death (5.2% vs 13.0%, P =0.02) and MI (1.0% vs 4.6%, P =0.03) but similar ST rates (0.7% vs. 0.9%, P =1.0)[48]
. Compared with PESs, in the Endeavor IV trial ZESs showed similar efficacy and safety[49]
. At five years, target lesion failure (17.2% vs. 21.1%, P =0.06) and ST (1.3% vs. 2.0%, P =0.42) were similar with ZESs and PESs[50]
. However, very late ST (0.4% vs. 1.8%, P =0.012) and late MI (1.3% vs. 3.5%, P =0.008) events were lower with ZESs compared with PESs.
The RESOLUTE All-comers and TWENTE trials compared R-ZESs with EESs. In the RESOLUTE All-comers trial, 2,292 patients were randomised 1:1 to ZESs or EESs[51]
. At five years, ZES-treated patients had similar rates of device oriented (17.0% vs. 16.2%, P =0.61), patient oriented composite end points (35.3% vs. 32.0%, P =0.11) and definite or probable ST (2.8% vs. 1.8%, P =0.12) compared with EES-treated patients. The TWENTE trial randomised 1,391 patients to ZESs or EESs[15]
. At one year, the incidence of definite or probable ST was similar between the two stent groups (0.9% vs 1.2%, P =0.59). ZESs were non-inferior to EESs for one-year target vessel failure, composite cardiac death, MI or clinically indicated target vessel revascularisation (P =0.001).
The Norwegian Coronary Stent Trial (NORSTENT) randomised 9,013 patients to either DESs or BMSs[52]
. In the DES arm, 83% stents were EESs while 13% were ZESs. Nearly two-thirds of patients had ACS and the mean DAPT duration was nine months. At six years, although DESs were associated with lower repeat revascularisation (16.5% vs. 19.8%, P <0.001), there was no difference in the primary end point of death or non-fatal MI (16.6% vs. 17.1%, P =0.66). Definite ST was low in both groups but appeared to be lower with DESs (0.8% with DESs vs. 1.2% with BMSs, P =0.0498).
Meta-analyses
Both patient level and network level meta-analyses have comprehensively analysed the data for DESs, comparing BMSs with first and second generation DESs. Palmerini et al. conducted a network meta-analysis from 51 randomised controlled trials and more than 50,000 patients to compare long-term outcomes with BMSs vs DESs[53]
. After a median four-year follow-up, all DESs were superior to BMSs for TVR. CoCr-EESs were associated with lower mortality, definite ST and MI compared with BMSs and first generation DESs, and lower TVR compared with BMSs, PESs and phosphorylcholine polymer ZESs. ZESs were associated with lower ST than SESs and lower MI than BMSs and PESs.
Valgimigli et al. confirmed these findings in a patient level meta-analysis of 4,896 patients from five randomised controlled trials. The authors observed that CoCr-EESs were associated with lower mortality, MI and definite ST compared with BMSs[54]
. These differences remained after multivariable adjustment for female gender, ACS, diabetes, use of glycoprotein IIb/IIIa inhibitors and duration of DAPT.
New generation devices
Bioresorbable scaffolds
The proposed benefits of bioresorbable devices are complete resorption of the scaffold over two years allowing normal vasomotion to return, absence of a permanent metallic cage, a potentially reduced need for prolonged DAPT, as well as lack of hindrance to future revascularisation with percutaneous or surgical methods[27]
. Bioresorbable scaffolds comprise a fully biodegradable jacket, either polymeric or metal. The ABSORB™ bioresorbable vascular scaffold (BVS) (Abbott Vascular, California, United States) and DESolve® scaffold (Elixir Medical, California, United States) are two available polymeric scaffolds. The ABSORB bioresorbable vascular scaffold (BVS) is composed of a polymer backbone of poly-L-lactic acid (PLLA) coated with a mixture of Poly-D,L lactide polymer and a limus drug[28]
.
Both registry data and randomised trials have assessed the safety and effectiveness of ABSORB stents. The ABSORB cohort A study enrolled 30 patients treated with BVS for de novo lesions <18mm in length[17]
. At six months, the angiographic in-stent loss was 0.44mm (0.35mm) because of mild stent shrinkage and neointimal hyperplasia[55]
. However, at two years there was no significant additional angiographic late lumen loss compared with six-month findings (0.48mm, 27% reduction in lumen size)[17]
. With respect to clinical end points, the two year MACE (composite cardiac death, MI or TLR) was 3.6%. The ABSORB cohort B trial enrolled 101 patients treated with the modified BVS device[56]
. On serial imaging at 6, 12, 24 and 36 months, late lumen loss remained unchanged between one and three years with 6% in-segment angiographic restenosis rate and no scaffold thrombosis. The clinical MACE rate at two years was 10.0%.
The ABSORB II trial randomised 501 stable patients undergoing PCI to BVSs or EESs[57]
. The co-primary end points were vasomotion and difference between minimum lumen diameter post-procedure and at three years. Acute lumen gain was lower for BVSs vs. EESs (1.15mm vs. 1.46mm, P <0.001). At three years, rates of angina were lower with BVSs (22% vs. 30%, P =0.04) but performance during maximum exercise and Seattle angina questionnaire scores were similar. There were no differences in one-year target lesion failure (5% with BVSs vs. 3% with EESs, P =0.35) or one-year patient-oriented clinical composite end point of all cause death, any MI or TVR (7% vs. 9%, P =0.47).
The ABSORB III trial randomised 2,008 patients with stable or unstable angina 2:1 to BVSs or EESs[20]
. The primary end point of one-year target lesion failure (composite cardiac death, target vessel MI, ischaemia driven target lesion revascularisation) occurred in 7.8% of the BVS group vs. 8.1% of the EES group (P =0.16 for superiority and P =0.007 for non-inferiority). Device thrombosis occurred in 1.5% of the BVS group vs. 0.7% of the EES group at one year (P =0.13). Similarly, the TROFI-II trial randomized 191 STEMI patients to BVSs or EESs[58]
. At six months, healing scores, based on uncovered or malapposed stent struts or intraluminal filling defects on optical coherence technology were similar for both device types (mean healing score 1.74 [SD 2.39] for BVSs vs. 2.80 [SD 4.44] for EESs, P <0.001 for non-inferiority). The device oriented end points were low in both groups (1.1% for BVSs vs. 0% for EESs), with one scaffold thrombosis in the BVS group and none in the EES group.
Other randomised trials, such as ABSORB Japan and ABSORB China, have also demonstrated similar efficacy and safety of BVSs compared with EESs[59],[60]
.
Despite this, there may be technical issues associated with BVSs consequent to thicker struts, risk of fracture from post-dilation and concerns regarding device breakage[61]
. Real world registry data for BVSs have also indicated concerns related to subacute and late ST with rates of 1.5% at 30 days and 2.1% at six months[62]
. A meta-analysis of six randomised trials and 3,738 patients comparing metallic EESs and EES BVSs showed similar risk of TLR, death and MI between the two stent groups[63]
. However, patients receiving BVSs had a higher risk of definite or probable ST than metallic EESs (odds ratio [OR] 1.99; 95% CI 1.00–3.98; P =0·05). The highest risk was in the first 30 days (OR 3.11; 95% CI 1.24–7.82; P =0.02).
The DESolve first-in-man trial examined the performance of the DESolveâ„¢ myolimus eluting polymeric scaffold (Elixir Medical, California, United States). At six months, late lumen loss was 0.19mm (SD 0.19) and at one year there was no scaffold thrombosis or scaffold related MACEs[64]
. The DESOlve Nx observational study enrolled 126 patients from 15 sites[65]
. The primary end point was six months late lumen loss which was 0.21 ± 0.32mm and two year MACE was 7.4% with no definite ST.
The DREAMS (drug-eluting absorbable metal scaffold) stent incorporates drug-eluting technology with a magnesium alloy metal bioresorbable stent (BRS). The BIOSOLVE I trial was a multicentre trial enrolling 46 patients treated with the paclitaxel eluting magnesium alloy BRS[29]
. At one year, the rate of target lesion failure was 7% with no cardiac death or scaffold thrombosis. In the BIOSOLVE II trial, first-in-man non-randomised trial enrolling 126 patients, second generation magnesium- SES (DREAMS-2G [Biotronik, Berlin, Germany]) showed low rates of neointimal hyperplasia (mean neointimal area 0.08mm2 at six months) and one-year target lesion failure (3.4%) with no ST events[21]
,[30]
. These studies indicate the feasibility of metal BRS as an alternative to polymeric scaffolds in patients undergoing PCI.
The Fantom® scaffold (Reva Medical, California, United States) is a tyrosine polymer based bioresorbable device with sirolimus eluting technology[66]
. The ongoing FANTOM II multicentre single arm trial will assess the safety and performance of this scaffold for clinical and device related primary end points at six months (clinicaltrials.gov NCT02539966).
Biodegradable polymer DES
Biodegradable polymer (BDP) DESs comprise a resorbable polymer but durable scaffold, wherein the polymer completely degrades over 4–9 months[22]
,[27],[67]
. This technology aims to mitigate the risk associated with polymer hypersensitivity and late ST and consequently the need for prolonged DAPT may be reduced. However, studies are somewhat conflicting on whether or not biolimus-eluting BDP stents are associated with lower inflammatory score and good endothelialisation patterns[68],[69]
. In a study of BESs compared with EESs, BESs were associated with higher incidence of malapposed struts and more struts with incomplete endothelialisation[69]
. There are also some concerns regarding stent fracture with BDP stents[70]
. However, clinical trial data are encouraging.
Biomatrixâ„¢ BA9 (Co-Cr BES)
The Leaders trial was a multicenter non-inferiority trial comparing the biodegradable polymer Biolimusâ„¢ A9 (BES) with first generation SES in 1,707 patients[71]
. At five years, the incidence of device oriented end points (composite cardiac death, MI or clinically indicated target lesion revascularisation) was 22.3% with BESs vs. 26.1% with SESs (P <0.0001 for non-inferiority). The patient-oriented end points were lower with BESs (35.1% vs. 40.4%, P =0.023), as were ST events (relative risk [RR] 0.20; 95% CI 0.06–0.71). From one to five years, biodegradable polymer BESs were associated with lower ST than SESs (0.7% vs 2.5%).
Nobori® (Co-Cr BES)
The Nobori 1 RCT compared biodegradable polymer Biolimus A9 stents to PESs in 363 patients randomised 2:1 across 29 centres[72]
. At five years, the incidence of ST was lower with BESs (0% vs. 3.2% for ST, P =0.014) but there were no differences in the rate of death or MI (10.9 vs.11.2%) or target lesion failure (9.2 vs. 10.4%)[73]
. In the NEXT trial, 3,235 patients across 98 centres in Japan were randomised to the Nobori BESs or EESs[74]
. At one year, the incidence of ST was similar between the two stent types (0.25% vs. 0.06%, P =0.18)[74]
. At three years, the incidence of MACEs was 9.9% with BESs vs. 10.3% with EESs (P <0.0001 for non-inferiority)[75]
. The COMPARE II trial randomised 2,707 patients 2:1 to Nobori BESs or EESs and showed similar incidence of the primary end point (cardiac death, non-fatal MI or target vessel revascularisation) at one year (5.2% vs. 4.8%, P <0.001 for non-inferiority)[76]
.
A meta-analysis of 9,114 patients compared BDP BESs (Nobori) to other durable polymer DESs and found comparable 12-month outcomes for TLR, ST, MI, death and MACEs[77]
.
SYNERGY (Pt-Cr EES)
The EVOLVE II RCT randomised 1,684 patients to either the Synergy biodegradable polymer EES or Promus durable polymer EES[78]
. At one year, MACEs occurred in 6.7% of Synergy stents vs. 6.5% of Promus stents (P <0.001 for non-inferiority). The rate of ST was similar for the two stent types – 0.4% vs. 0.6%, respectively (P =0.50).
Ultimaster® (Co-Cr SES)
The CENTURY II trial randomised 1,123 patients to the Ultimaster biodegradable polymer SES (Terumo, Leuven, Belgium) or to the permanent polymer Everolimus XIENCE stent (Abbott Vascular, California, United States)[79]
. At nine months, ST was low in both arms (0.9%). The composite of death or MI (2.9% vs. 3.8%, P =0.40) and TVR (4.5% vs. 4.2%, P =0.77) was similar between the groups. The Ultimaster stent was shown to be non-inferior to XIENCE for the primary end point of death, MI or TVR.
Orsiro (Co-Cr SES)
The Orsiro stent is an ultrathin SES with passive and active components. The PROBIO® passive coating envelops the stent and minimises interaction between the metal stent and surrounding tissue[22]
. It is asymmetrical with a thicker abluminal side allowing greater drug concentration on this side. The active coating is BIOlute, which contains a highly biocompatible and biodegradable polymer. BIOFLOW III registry data demonstrates that Orsiro is safe and effective in complex lesions with low TLR rates[80]
. The SORT OUT VII trial randomised 2,525 patients to the Orsiro SES or Nobori BES. At 12 months, target lesion failure occurred in 3.8% vs. 4.6% (P <0.0001 for non-inferiority)[81]
. Patients receiving the Orsiro stent had a lower rate of ST (0.4% vs. 1.2%, P =0.03) compared with Nobori BES-treated patients. The BIOSCIENCE trial randomised 2,119 patients with ACS or stable angina to the Orsiro stent or an EES[82]
. At 12 months, target lesion failure (10.4% vs. 10.5%, P =0.98) and ST rates (3.9% vs. 4.9%, P =0.29) were similar.
DESyne® (Co-Cr Novolimus, Elixir Medical, California, United States)
The EXCELLA II trial randomised 210 patients 2:1 to the biodegradable coating technology NES (n=139) or Endeavor ZES (n=71)[24]
. At five years, the NES group had lower incidence of device (7.9% vs. 19.7%, P =0.02) and patient-oriented composite (23.7% vs. 40.8%, P =0.016) endpoints without a difference in definite ST (1.4% vs. 1.4%) or cardiac death.
Yukon stent (Translumina Therapeutics, New Delhi, India)
The Yukon stent comprises a microporous sirolimus stent with a biodegradable polymer. The ISAR-TEST trial demonstrated similar efficacy and safety for up to five years with the Yukon stent compared with both EESs and SESs[32]
. A total of 2,603 patients were randomised to the Yukon® PC Choice stent (n=1,299) or XIENCE EES (n=652) or to durable polymer Cypher SES (n=652). The primary endpoint of five-year composite cardiac death, target vessel MI or target lesion revascularisation were similar with the Yukon stent vs. XIENCE (20.5% vs. 19.5%) and Cypher vs. XIENCE (23.5% vs. 19.5%). Analogously, there were no differences in ST between the groups, but numerically higher rates were observed with Cypher stents (1.2% with Yukon stents, 1.4% with XIENCE EESs, 2.4% with Cypher SESs).
The LIPSIA-Yukon trial compared the Yukon Choice stent with Taxus Liberté PES in a randomised manner in 240 patients with diabetes[83]
. The primary end point was in-stent late lumen loss at nine months. The Yukon stent was not shown to be non-inferior to the PES (late lumen loss 0.63 ± 0.62mm with the Yukon Choice and 0.45 ± 0.60 with the PES). However, at five years, outcomes were similar between the two stent groups[84]
.
Polymer free stents
The multicentre first-in-man Biofreedom clinical trial randomised 182 patients 1:1:1 to the standard dose BioFreedom drug coated stent (BFD DCS) or low dose BFD DCS or PES[85]
. The BFD DCS has a microstructured abluminal surface that facilitates adhesion and release of biolimus A9. The standard dose, but not low dose BFD DCS, was non-inferior to PES for late lumen loss (0.17mm vs. 0.35mm vs. 0.22mm), a surrogate for TLR. However, at five years there were no differences in clinical event rates between the groups and no ST events in any group.
In the LEADERS FREE trial, for the first time patients at high bleeding risk were randomised to polymer-free BESs or similar BMSs[31]
. At 390 days, the primary safety end point of death, MI or ST occurred in 9.4% of the BES group vs. 12.9% in the BMS group (risk difference -3.6%, P =0.005). Any TLR occurred in 5.1% of the BES group vs. 9.8% of the BMS group (risk difference -4.8%, P <0.001). The incidence of ST (2.2% vs. 2.0%, P =0.75) and major bleeding was similar in both groups (7.2% vs. 7.3%, P =0.96).
Endothelial progenitor cell technology stents
The Genousâ„¢ Bioengineered R stentâ„¢ (OrbusNeich, Wanchai, Hong Kong) comprises a metal scaffold associated with endothelial progenitor cell (EPC) capture technology – immobilised antibodies to bind to endothelial cell surface antigens[41]
. The HEALING series of studies showed this technology to be non-inferior to PES[86]
. However, the success of this device may be dependent on the total number of healthy circulating EPCs. Interestingly, the total number of EPCs may be increased by use of statins[87]
. The COMBO stent (OrbusNeich, Wanchai, Hong Kong) combines the EPC capture technology luminally with the sirolimus coated abluminal surface[19]
. In the Remedee trial, the COMBO stent was non-inferior to EES for angiographic in-stent late lumen loss at nine months (0.39mm ± 0.45mm vs. 0.44mm ± 0.56mm, P =0.0012 for non-inferiority)[36]
. There was no difference in clinical outcomes at 12 months.
Societal guidelines and DES use in special populations
The American College of Cardiology/American Heart Association (ACC/AHA) guidelines recommend BMS use in cases when patients are unable to tolerate or comply with DAPT, in case of anticipated surgery within six months or in high-risk cases for bleeding[1],[88]
. Similarly, the European Society of Cardiology (ESC) guidelines recommend selection of DESs in stable angina patients, in whom there is no contraindication to prolonged DAPT. Further, the most recent DESs with thinner struts and biocompatible or biodegradable polymers are recommended in stable coronary artery disease with no contraindication to DAPT[89]
.
Certain patient subsets warrant special consideration for stent selection. The clinical trial data in some of these high-risk groups are explained below.
Diabetes mellitus
In patients with diabetes mellitus (DM), extensive disease and need for repeat revascularisation are pertinent issues. In a registry of 21,045 patients, 29% (n=5,051) of whom were diabetic patients treated between 2003 and 2004, DES use versus BMS use was associated with lower unadjusted risk of all adverse events. In a propensity score adjusted model comparing 1,476 DES-treated DM patients with 1,476 BMS-treated DM patients, DES use was associated with lower three-year risk of death (risk difference -3.2%, P =0.02), MI (risk difference -3.3%, P =0.02) and revascularisation (risk difference -5.4%, P <0.001) compared with BMS[90]
. In a mixed treatment meta-analysis including 22,844 patient-years, all DES types reduced the risk of TLR in DM patients compared with BMSs, however EESs were the most superior DESs with 87% probability[91]
. Furthermore, DESs were not associated with a higher risk of two-year ST compared with BMSs, however, EESs were associated with the lowest rates of ST with 62% probability.
Several studies have compared different DES types in a diabetic patient population. In the ESSENCE-DIABETES trial, patients were randomised to treatment with EESs (n=149) or SESs (n=151). EES-treated patients were found to have similar rates of major adverse revascularisation rates compared with SES-treated patients[92]
. In the SPIRIT V diabetic trial, 324 patients were randomised 2:1 between EESs (n=218) and PESs (n=106). At one year, there was no difference in the rate of composite death, MI or revascularisation (16.3% vs. 16.4%) or ST (0% vs. 2.0%, P =0.11)[93]
. A pooled analysis of the SPIRIT and COMPARE trials (EES vs. PES), comprising 6,780 patients and 27.6% patients with diabetes, demonstrated an interaction between diabetes and type of stent for MI (P =0.01), ST (P =0.0006) and revascularisation (P =0.02); although EESs were superior to PESs in non-diabetic patients, no difference was found in these major endpoints among diabetic patients[94]
. By contrast, the Taxus Element versus XIENCE Prime in a Diabetic Population (TUXEDO) multicentre RCT conducted across 46 centres in India demonstrated for the first time the superiority of EESs compared with PESs among patients with diabetes[95]
. A total of 1,851 patients were randomised 1:1 to EESs (n=916) or PESs (n=914). At one year, EESs were found to be superior to PESs for the primary end point of target vessel failure, defined as a composite of cardiac death, target-vessel myocardial infarction, or ischaemia-driven TVR (2.9% vs. 5.6%, P =0.005).
In a pooled evaluation of the Endeavor III and IV trials, comparing the Endeavor-ZES (n=337) with first generation DESs (n=32 SES, n=236 PES) in patients with diabetes, five-year outcomes were similar between the groups for the incidence of target vessel failure (20.2% vs. 26.9%, P =0.065), however, the incidence of MACEs (17.7% vs. 26.6%, P =0.012), death (7.6% vs. 15.0%, P =0.004) and MI (1.3% vs. 5.1%, P =0.011) were significantly lower in diabetic patients treated with E-ZESs[96]
. The rates of revascularisation (11.2% with E-ZESs vs. 12.0% with other DESs, P =0.800) and ST (1.2% with E-ZESs vs. 0.8% with other DESs, P =0.59) were not different between the groups. In a propensity score adjusted analysis (n=1,855 patients with diabetes) from the EXCELLENT and Resolute-Korea observational studies, Park et al. found no differences in outcomes between EES (n=934) and Resolute-ZES (n=934) treated diabetic patients for one-year target lesion failure (3.9% vs. 3.4%, P =0.86), MACEs (9.4% vs. 8.6%, P =0.73) or individual end points[97]
.
Similarly, in a propensity score matched analysis of ABSORB and SPIRIT trial patients, diabetic patients receiving EES BVSs had a similar incidence of device oriented end points (cardiac death, target vessel MI or TLR) to those receiving metallic EESs (3.9% for BVSs vs 6.4% for EESs, P =0.38)[98]
. Furthermore, diabetic and non-diabetic patients treated with EES BVSs demonstrated similar rates of the device oriented composite end point (3.7% vs. 5.1%, P =0.64). Multicentre registry data for biodegradable polymer DESs in DM (n=334) suggest a very low MACE rate (composite of death, MI or TVR) of 1.27% at 12 months[99]
.
Therefore, second generation and novel DESs have produced excellent short-term and long-term outcomes in patients with DM. The specific influence of insulin treated DM on long-term outcomes warrants examination in future studies.
Chronic kidney disease
Patients with chronic kidney disease (CKD) have greater systemic comorbidities and extensive anatomical disease including longer lesion lengths and smaller lesion diameters, associated with a higher thrombotic risk compared with patients without CKD[100],[101]
. In a meta-analysis, Bangalore et al. assessed the outcomes in 83,332 patients and reported lower death, cardiac death, MI and ST in CKD patients with DESs compared with BMSs[102]
. The BASKET-PROVE investigators showed that both patients with and without CKD uniformly benefited from DESs compared with BMSs[103]
. Green et al. evaluated NHLBI data and demonstrated that the benefit with respect to lower need for revascularisation with DESs was attenuated in CKD patients[104]
. Regardless of stent type, CKD patients had worse outcomes than non-CKD patients. Conversely, in a patient level analysis from 26 randomised trials that included women treated with DESs, impaired renal function was shown to be an independent predictor of MACEs, MI and death but not TLR[105]
. Furthermore new-generation DESs were associated with reduced incidence of cardiac death, MI or ST compared with old-generation DESs, without evidence of interaction by CKD status.
In addition to high ischaemic risk, CKD patients also have a higher risk of bleeding compared with non-CKD patients[101]
and prolonged DAPT durations may result in greater morbidity in this patient group. The selection of stent type and antiplatelet strategy in CKD patients should therefore be based on the balance of ischaemic and bleeding risks in individual patients.
STEMI
The safety and efficacy of DESs have also been specifically examined in acute myocardial infarction (AMI) and STEMI patients.
In the Trial to Assess the Use of the Cypher Stent in Acute Myocardial Infarction Treated with Balloon Angioplasty (TYPHOON) trial, SESs were shown to be superior to BMSs in the reduction of TVR but not death or MI[106]
. Conversely, in the Paclitaxel-Eluting Stent versus Conventional Stent in Myocardial Infarction with ST-Segment Elevation (PASSION) trial, PESs were similar in efficacy and safety to BMSs for all ischaemic end points[107]
. A meta-analysis, including these two trials, showed that first generation DESs resulted in better outcomes than BMSs with respect to TVR (hazard ratio [HR] 0.38; 95% CI 0.29–0.50) but similar rates of death, ST and recurrent MI[108]
.
With respect to second generation DESs, the EXAMINATION randomised trial demonstrated efficacy of EESs compared with BMSs during two-year follow-up after STEMI[109]
. The incidence of TLR (2.9% vs 5.6%, P =0.009) and ST (0.8% vs 2.1%, P =0.03) was lower with EESs compared with BMSs.
Palmerini et al. performed a network level meta-analysis of 22 trials and 12,453 STEMI patients[110]
. Co-Cr-EESs were found to be superior to BMSs for one-year MACEs (death, MI, ST); the differences for ST between the two stent groups were noted as early as 30 days and sustained at two years. CoCr-EESs, PESs, SESs but not ZESs were associated with lower one-year TVR compared with BMS. Similarly, compared with BMSs, SESs were associated with lower one-year death or MI; compared with PESs, SESs were associated with lower TVR. Compared with PESs, CoCr-EESs were associated with lower one-year ST.
The Swedish SCAAR registry evaluated outcomes for old DESs vs. new DESs vs. BMSs in STEMI patients[111]
. The investigators examined 34,147 patients between 2007 and 2013, of whom 14% received new DESs, 12.5% received old DESs and the majority received BMSs. Patients treated with new DESs showed lower incidence of early/late ST than patients treated with BMSs. The risk of very late ST was comparable between new DESs and BMSs up to three years of follow-up, whereas old DESs were associated with an increased risk of very late ST.
The BVS-Examination STEMI study compared treatment with EES BVSs with metallic EESs and to BMSs in 870 STEMI patients[112]
. The one-year device oriented composite end point of cardiac death, target vessel MI or TVR was similar between the groups (4.1% with BVS vs. 4.1% with EES, P =0.994 vs. 5.9% with BMS, P =0.306), but the incidence of ST was numerically higher with BVSs (2.4% with BVS vs. 1.4% with EES, P =0.948, vs. 1.7% with BMS, P =0.825). The TROFI II trial also compared EES BVSs with metallic EESs in STEMI patients and found no angiographic differences at nine months[58]
. The device oriented composite end point was comparably low between the groups (1.1% vs. 0.0%), as was the incidence of ST (1 ST with BVSs and none with EESs).
Patients at high bleeding risk
The LEADERS FREE trial randomised patients at high bleeding risk to receive treatment with polymer free BioFreedom BES or similar BMS[31]
. Nearly two-thirds of enrolled patients were aged ≥75 years. At one year, BESs were not only associated with lower TLR than BMSs, but there was also a similar incidence of ST and major bleeding. These data are encouraging for the treatment of high bleeding risk patients with novel DESs.
In the ZEUS (Zotarolimus-eluting Endeavor sprint stent in Uncertain DES candidates) trial[113]
, investigators randomised 1,606 patients with high thrombotic or bleeding risk but low restenotic risk to either BMSs or ZESs with a biocompatible polymer and fast elution characteristics. Patients were given DAPT for a median of 32 days. At one year, the incidence of MACEs (composite death, MI or TVR) occurred in 17.5% patients on ZESs compared with 22.1% patients on BMSs (P =0.011). The authors concluded that in stable or unstable patients with high thrombotic/bleeding risk with uncertain indication for DESs, ZESs may be a suitable option allowing for short DAPT duration post PCI.
The selection of DES type and the ideal antithrombotic strategy in patients needing chronic oral anticoagulation can be particularly challenging. In the e-SELECT SES observational registry, only 3.9% (296/7651) of PCI patients were discharged on vitamin K antagonists[114]
. However, compared with their counterparts, patients on vitamin K antagonists had greater incidence of bleeding events following DES implantation. The What is the Optimal antiplatElet and anticoagulant therapy in patients with oral anticoagulation and coronary StenTing (WOEST) trial was the first to randomise PCI patients eligible for chronic oral anticoagulation to treatment with triple therapy (acetyl salicylic acid, clopidogrel and warfarin) or dual therapy with warfarin and clopidogrel. Nearly two-thirds of enrolled patients had atrial fibrillation at baseline and two-thirds of all patients received DESs[115]
. The primary objective of the study was difference in any bleeding between triple therapy and dual therapy groups, defined using the Bleeding Academic Research consortium bleeding scale. The triple therapy group had greater incidence of the primary end point (22.1% vs. 17.1%; P <0.0001) but there was no difference between the two groups for ischaemic outcomes[115]
.
Similarly, elderly patients undergoing PCI have greater comorbidities at baseline and a higher risk of bleeding. Consequently, selection of therapies requires more careful attention. The e-SELECT SES registry examined outcomes in 675 octogenarian patients and 14,472 non-octogenarian patients undergoing PCI with SESs[116]
. The incidence of cardiac death, MI, ST and major bleeding was higher in octogenarians compared with non-octogenarians, however, there were no differences in the incidence of TLR.
DAPT duration with DES
There is ongoing controversy about the optimal DAPT duration in patients treated with DESs. The DAPT trial suggested that patients treated with prolonged DAPT for 30 months compared with 12 months had a lower risk of ST and MI, at the expense of greater bleeding[117]
. However, in a meta-analysis of DAPT trials, short compared with prolonged DAPT was not associated with increased risk of ST in patients treated with new-generation DESs[118]
.
Two recent risk scores reported on the risk factors that warrant consideration for decision-making on prolonged DAPT duration in DES treated patients[119]
,[120]
. These included baseline characteristics, such as patient age, prior MI or PCI, current smoking, diabetes, heart failure, renal dysfunction, ACS and procedural characteristics including the number of vessels treated and stent diameter <3mm.
The current ACC/AHA and ESC guidelines recommend at least six months of DAPT in patients treated with DES[88]
,[121]
and 12 months of DAPT in patients with ACS, if tolerated.
Summary
DESs have overcome the limitations of BMSs and dramatically reduced the need for revascularisation to less than 10% in several randomised trials. The complication of late and very late ST associated with first generation DESs (the result of polymer hypersensitivity) has also been mitigated with the use of biocompatible polymers and thinner struts in second generation DESs. Consistently, clinical trial data indicate long-term ST to be <1%. Furthermore, bioresorobable scaffolds, biodegradable polymer DESs and polymer free drug coated stents are associated with low clinical event rates and have been shown to be non-inferior to metallic stents with durable polymer. The aim of these novel stent designs is to further decrease the required duration of DAPT. Indeed, these technologies stand to reestablish the role and need for BMSs in current practice. Concurrent with the improvements in stent manufacturing, attention to optimal implantation techniques, including judicious use of intra-coronary imaging, remain crucial to reduce risk associated with stent undersizing, underexpansion and malapposition. Moreover, tailored patient therapies with respect to choice and intensity of P2Y12 inhibition and DAPT duration are important. Special considerations are warranted in high-risk populations with diabetes, renal dysfunction, STEMI, patients on chronic oral anticoagulation and elderly patients. Risk scores may be useful to guide decision-making on DAPT duration in individual patients.
Kyra Martin, Jaya Chandrasekhar and Roxana Mehran — Icahn School of Medicine at Mount Sinai, New York, United States.
Financial and conflicts of interest disclosure: The authors have no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or material discussed in this manuscript. No writing assistance was utilised in the production of this manuscript.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] Levine GN, Bates ER, Blankenship JC et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011;124(23):e574-651. doi: 10.1161/cir.0b013e31823ba622
[2] Riley RF, Don CW, Powell W, Maynard C & Dean LS. Trends in coronary revascularization in the United States from 2001 to 2009: recent declines in percutaneous coronary intervention volumes. Circ Cardiovasc Qual Outcomes 2011;4(2):193–197. doi: 10.1161/circoutcomes.110.958744
[3] O’Gara PT, Kushner FG, Ascheim DD et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the American College of Emergency Physicians and Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv 2013;82(1):E1–27. doi: 10.1002/ccd.24776
[4] Hamm CW, Bassand JP, Agewall S et al. [ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC)]. G Ital Cardiol (Rome) 2012;13(3):171–228. doi: 10.1714/1038.11322
[5] Levine GN, Bates ER, Blankenship JC et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011;124(23):2574–2609. doi: 10.1161/cir.0b013e31823a5596
[6] King SB 3rd. The development of interventional cardiology. J Am Coll Cardiol 1998;31(4 Suppl B):64B–88B.PMID: 9530288
[7] Morice MC, Serruys PW, Sousa JE et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med 2002;346(23):1773–1780. doi: 10.1056/nejmoa012843
[8] Weisz G, Leon MB, Holmes DR Jr. et al. Two-year outcomes after sirolimus-eluting stent implantation: results from the Sirolimus-Eluting Stent in de Novo Native Coronary Lesions (SIRIUS) trial.J Am Coll Cardiol 2006;47(7):1350–1355. doi: 10.1016/j.jacc.2005.11.077
[9] Holmes DR, Jr., Kereiakes DJ, Laskey WK et al. Thrombosis and drug-eluting stents: an objective appraisal.J Am Coll Cardiol 2007;50(2):109–118. doi: 10.1016/j.jacc.2007.04.032
[10] Weisz G, Leon MB, Holmes DR Jr. et al. Five-year follow-up after sirolimus-eluting stent implantation results of the SIRIUS (Sirolimus-Eluting Stent in De-Novo Native Coronary Lesions) Trial. J Am Coll Cardiol 2009;53(17):1488–1497. doi: 10.1016/j.jacc.2009.01.050
[11] Stone GW, Ellis SG, Colombo A et al. Long-term safety and efficacy of paclitaxel-eluting stents final 5-year analysis from the TAXUS Clinical Trial Program. JACC Cardiovasc Interv 2011;4(5):530–542. doi: 10.1016/j.jcin.2011.03.005
[12] Morice MC, Serruys PW, Barragan P et al. Long-term clinical outcomes with sirolimus-eluting coronary stents: five-year results of the RAVEL trial. J Am Coll Cardiol 2007;50(14):1299–1304. doi: 10.1016/j.jacc.2007.06.029
[13] Stone GW, Rizvi A, Newman W et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med 2010;362(18):1663–1674. doi: 10.1056/nejmoa0910496
[14] Serruys PW, Silber S, Garg S et al. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med 2010;363(2):136–146. doi: 10.1056/nejmoa1004130
[15] von Birgelen C, Basalus MW, Tandjung K et al. A randomized controlled trial in second-generation zotarolimus-eluting Resolute stents versus everolimus-eluting Xience V stents in real-world patients: the TWENTE trial. J Am Coll Cardiol 2012;59(15):1350–1361. doi: 10.1016/j.jacc.2012.01.008
[16] Panoulas VF, Mastoris I, Konstantinou K et al. Everolimus-eluting stent platforms in percutaneous coronary intervention: comparative effectiveness and outcomes. Med Devices (Auckl) 2015;8:317–329. doi: 10.2147/mder.s66360
[17] Serruys PW, Ormiston JA, Onuma Y et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet 2009;373(9667):897–910. doi: 10.1016/s0140-6736(09)60325-1
[18] Schurtz G, Delhaye C, Hurt C et al. Biodegradable polymer Biolimus-eluting stent (Nobori®) for the treatment of coronary artery lesions: review of concept and clinical results. Med Devices (Auckl) 2014;7:35–43. doi: 10.2147/mder.s44051
[19] Granada JF, Inami S, Aboodi MS et al. Development of a novel prohealing stent designed to deliver sirolimus from a biodegradable abluminal matrix. Circ Cardiovasc Interv 2010;3(3):257–266. doi: 10.1161/circinterventions.109.919936
[20] Ellis SG, Kereiakes DJ, Metzger DC et al. Everolimus-eluting bioresorbable scaffolds for coronary artery disease. N Engl J Med 2015;373(20):1905–1915. doi: 10.1056/nejmoa1509038
[21] Haude M, Ince H, Abizaid A et al. Sustained safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de novo coronary lesions: 12-month clinical results and angiographic findings of the BIOSOLVE-II first-in-man trial. Eur Heart J 2016;37(35):2701–2709. doi: 10.1093/eurheartj/ehw196
[22] Lam MK, Sen H, Tandjung K, et al. Comparison of 3 biodegradable polymer and durable polymer-based drug-eluting stents in all-comers (BIO-RESORT): rationale and study design of the randomized TWENTE III multicenter trial. Am Heart J 2014;167(4):445–451. doi: 10.1016/j.ahj.2013.11.014
[23] Daemen J & Serruys PW. Drug-eluting stent update 2007: part I. A survey of current and future generation drug-eluting stents: meaningful advances or more of the same? Circulation 2007;116(3):316–328. doi: 10.1161/circulationaha.106.621342
[24] Iqbal J, Verheye S, Abizaid A et al. DESyne novolimus-eluting coronary stent is superior to Endeavor zotarolimus-eluting coronary stent at five-year follow-up: final results of the multicentre EXCELLA II randomised controlled trial. EuroIntervention 2015;11(6):pii:20150130-01. doi: 10.4244/EIJY15M10_04
[25] Luscher TF, Steffel J, Eberli FR et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation 2007;115(8):1051–1058. doi: 10.1161/circulationaha.106.675934
[26] Garg S & Serruys PW. Coronary stents: looking forward. J Am Coll Cardiol 2010;56(10 Suppl):S43–78. doi: 10.1016/j.jacc.2010.06.008
[27] Serruys PW, Garcia-Garcia HM & Onuma Y. From metallic cages to transient bioresorbable scaffolds: change in paradigm of coronary revascularization in the upcoming decade? Eur Heart J 2012;33(1):16–25b. doi: 10.1093/eurheartj/ehr384
[28] Serruys PW, Onuma Y, Ormiston JA et al. Evaluation of the second generation of a bioresorbable everolimus drug-eluting vascular scaffold for treatment of de novo coronary artery stenosis: six-month clinical and imaging outcomes. Circulation 2010;122(22):2301–2312. doi: 10.1161/circulationaha.110.970772
[29] Haude M, Erbel R, Erne P et al. Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12 month results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. Lancet 2013;381(9869):836–844. doi: 10.1016/s0140-6736(12)61765-6
[30] Haude M, Ince H, Abizaid A et al. Safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de-novo coronary artery lesions (BIOSOLVE-II): 6 month results of a prospective, multicentre, non-randomised, first-in-man trial. Lancet 2016;387(10013):31–39. doi: 10.1016/s0140-6736(15)00447-x
[31] Urban P, Meredith I, Abizaid A et al. for the LEADERS FREE Investigators. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med 2015;373:2038–2047. doi: 10.1056/nejmoa1503943
[32] Kufner S, Byrne RA, Valeskini M et al. Five-year outcomes from a trial of three limus-eluting stents with different polymer coatings in patients with coronary artery disease: final results from the ISAR-TEST 4 randomised trial. EuroIntervention 2016;11(12):1372–1377. doi: 10.4244/eijy14m11_02
[33] Claessen BE, Henriques JP & Dangas GD. Clinical studies with sirolimus, zotarolimus, everolimus, and biolimus A9 drug-eluting stent systems. Curr Pharm Des. 2010;16(36):4012–4024. doi: 10.2174/138161210794454941
[34] Meredith IT, Verheye S, Dubois CL et al. Primary endpoint results of the EVOLVE trial: a randomized evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent. J Am Coll Cardiol 2012;59(15):1362–1370. doi: 10.1016/j.jacc.2011.12.016
[35] Abizaid A & Costa JR, Jr. New drug-eluting stents: an overview on biodegradable and polymer-free next-generation stent systems. Circ Cardiovasc Interv 2010;3(4):384–393. doi: 10.1161/circinterventions.109.891192
[36] Haude M, Lee SW, Worthley SG et al. The REMEDEE trial: a randomized comparison of a combination sirolimus-eluting endothelial progenitor cell capture stent with a paclitaxel-eluting stent.JACC Cardiovasc Interv 2013;6(4):334–343. doi: 10.1016/j.jcin.2012.10.018
[37] Holmes DR Jr., Leon MB, Moses JW et al. Analysis of 1-year clinical outcomes in the SIRIUS trial: a randomized trial of a sirolimus-eluting stent versus a standard stent in patients at high risk for coronary restenosis. Circulation 2004;109(5):634–640. doi: 10.1161/01.cir.0000112572.57794.22
[38] Schampaert E, Moses JW, Schofer J et al. Sirolimus-eluting stents at two years: a pooled analysis of SIRIUS, E-SIRIUS, and C-SIRIUS with emphasis on late revascularizations and stent thromboses. Am J Cardiol 2006;98(1):36–41. doi: 10.1016/j.amjcard.2006.01.049
[39] Silber S, Colombo A, Banning AP et al. Final 5-year results of the TAXUS II trial: a randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for de novo coronary artery lesions. Circulation 2009;120(15):1498–1504. doi: 10.1161/circulationaha.109.849877
[40] Virmani R, Guagliumi G, Farb A et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation 2004;109(6):701–705. doi: 10.1161/01.cir.0000116202.41966.d4
[41] Daemen J & Serruys PW. Drug-eluting stent update 2007: part II: Unsettled issues. Circulation 2007;116(8):961–968. doi: 10.1161/circulationaha.107.691451
[42] Kirtane AJ & Stone GW. How to minimize stent thrombosis. Circulation 2011;124(11):1283–1287. doi: 10.1161/circulationaha.110.976829
[43] Yamaji K, Raber L, Zanchin T et al. Ten-year clinical outcomes of first-generation drug-eluting stents: the Sirolimus-Eluting vs. Paclitaxel-Eluting Stents for Coronary Revascularization (SIRTAX) VERY LATE trial. Eur Heart J 2016. doi: 10.1093/eurheartj/ehw343
[44] Gada H, Kirtane AJ, Newman W et al. 5-year results of a randomized comparison of XIENCE V everolimus-eluting and TAXUS paclitaxel-eluting stents: final results from the SPIRIT III trial (clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions). JACC Cardiovasc Interv 2013;6(12):1263–1266. doi: 10.1016/j.jcin.2013.07.009
[45] Stone GW, Rizvi A, Sudhir K et al. Randomized comparison of everolimus- and paclitaxel-eluting stents. 2-year follow-up from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) IV trial. J Am Coll Cardiol 2011;58(1):19–25. doi: 10.1016/j.jacc.2011.02.022
[46] Smits PC, Vlachojannis GJ, McFadden EP et al. Final 5-Year Follow-Up of a Randomized Controlled Trial of Everolimus- and Paclitaxel-Eluting Stents for Coronary Revascularization in Daily Practice: The COMPARE Trial (A Trial of Everolimus-Eluting Stents and Paclitaxel Stents for Coronary Revascularization in Daily Practice). JACC Cardiovasc Interv 2015;8(9):1157–1165. doi: 10.1016/j.jcin.2015.03.028
[47] Fajadet J, Wijns W, Laarman GJ et al. Long-term follow-up of the randomised controlled trial to evaluate the safety and efficacy of the zotarolimus-eluting driver coronary stent in de novo native coronary artery lesions: five year outcomes in the ENDEAVOR II study. EuroIntervention 2010;6(5):562–567. doi: 10.4244/eijv6i5a95
[48] Kandzari DE, Mauri L, Popma JJ et al. Late-term clinical outcomes with zotarolimus- and sirolimus-eluting stents. 5-year follow-up of the ENDEAVOR III (A Randomized Controlled Trial of the Medtronic Endeavor Drug [ABT-578] Eluting Coronary Stent System Versus the Cypher Sirolimus-Eluting Coronary Stent System in De Novo Native Coronary Artery Lesions). JACC Cardiovasc Interv 2011;4(5):543–550. doi: 10.1016/j.jcin.2010.12.014
[49] Leon MB, Mauri L, Popma JJ et al. A randomized comparison of the Endeavor zotarolimus-eluting stent versus the TAXUS paclitaxel-eluting stent in de novo native coronary lesions 12-month outcomes from the ENDEAVOR IV trial. J Am Coll Cardiol 2010;55(6):543–554. doi: 10.1016/j.jacc.2009.08.067
[50] Kirtane AJ, Leon MB, Ball MW et al. The “final” 5-year follow-up from the ENDEAVOR IV trial comparing a zotarolimus-eluting stent with a paclitaxel-eluting stent. JACC Cardiovasc Interv 2013;6(4):325–333. doi: 10.1016/j.jcin.2012.12.123
[51] Iqbal J, Serruys PW, Silber S et al. Comparison of zotarolimus- and everolimus-eluting coronary stents: final 5-year report of the RESOLUTE all-comers trial. Circ Cardiovasc Interv 2015;8(6):e002230. doi: 10.1161/circinterventions.114.002230
[52] Bonaa KH, Mannsverk J, Wiseth R et al. Drug-eluting or bare-metal stents for coronary artery disease. N Engl J Med 2016;375(13):1242–1252. doi: 10.1056/nejmoa1607991
[53] Palmerini T, Benedetto U, Biondi-Zoccai G et al. Long-term safety of drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol 2015;65(23):2496–2507. doi: 10.1016/j.jacc.2015.04.017
[54] Valgimigli M, Sabate M, Kaiser C et al. Effects of cobalt-chromium everolimus eluting stents or bare metal stent on fatal and non-fatal cardiovascular events: patient level meta-analysis. BMJ 2014;349:g6427. doi: 10.1136/bmj.g6427
[55] Ormiston JA, Serruys PW, Regar E et al. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet 2008;371(9616):899–907. doi: 10.1016/s0140-6736(08)60415-8
[56] Serruys PW, Onuma Y, Garcia-Garcia HM et al. Dynamics of vessel wall changes following the implantation of the absorb everolimus-eluting bioresorbable vascular scaffold: a multi-imaging modality study at 6, 12, 24 and 36 months. EuroIntervention 2014;9(11):1271–1284. doi: 10.4244/eijv9i11a217
[57] Serruys PW, Chevalier B, Dudek D et al. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet 2015;385(9962):43–54. doi: 10.1016/s0140-6736(14)61455-0
[58] Sabate M, Windecker S, Iniguez A et al. Everolimus-eluting bioresorbable stent vs. durable polymer everolimus-eluting metallic stent in patients with ST-segment elevation myocardial infarction: results of the randomized ABSORB ST-segment elevation myocardial infarction-TROFI II trial. Eur Heart J 2016;37(3):229–240. doi: 10.1093/eurheartj/ehv500
[59] Kimura T, Kozuma K, Tanabe K et al. A randomized trial evaluating everolimus-eluting Absorb bioresorbable scaffolds vs. everolimus-eluting metallic stents in patients with coronary artery disease: ABSORB Japan. Eur Heart J 2015;36(47):3332–3342. doi: 10.1093/eurheartj/ehv435
[60] Gao R, Yang Y, Han Y et al. Bioresorbable vascular scaffolds versus metallic stents in patients with coronary artery disease: ABSORB China Trial. J Am Coll Cardiol 2015;66(21):2298–2309. doi: 10.1016/j.jacc.2015.09.054
[61] Foin N, Lee R, Mattesini A et al. Bioabsorbable vascular scaffold overexpansion: insights from in vitro post-expansion experiments. EuroIntervention 2016;11(12):1389–1399. doi: 10.4244/eijy15m07_02
[62] Capodanno D, Gori T, Nef H et al. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice: early and midterm outcomes from the European multicentre GHOST-EU registry. EuroIntervention 2015;10(11):1144–1153. doi: 10.4244/eijy14m07_11
[63] Cassese S, Byrne RA, Ndrepepa G et al. Everolimus-eluting bioresorbable vascular scaffolds versus everolimus-eluting metallic stents: a meta-analysis of randomised controlled trials. Lancet 2015;387(10018):537–544. doi: 10.1016/s0140-6736(15)00979-4
[64] Verheye S, Ormiston JA, Stewart J et al. A next-generation bioresorbable coronary scaffold system: from bench to first clinical evaluation: 6- and 12-month clinical and multimodality imaging results. JACC Cardiovasc Interv 2014;7(1):89–99. doi: 10.1016/j.jcin.2013.07.007
[65] Abizaid A, Costa RA, Schofer J et al. Serial multimodality imaging and 2-year clinical outcomes of the novel DESolve novolimus-eluting bioresorbable coronary scaffold system for the treatment of single de novo coronary lesions. JACC Cardiovasc Interv 2016;9(6):565–574. doi: 10.1016/j.jcin.2015.12.004
[66] Reva Medical Inc. Bioresorbable coronary scaffolds. Available at: http://www.teamreva.com/coronary-stent.shtml (accessed October 2016)
[67] Garg S, Sarno G, Serruys PW et al. The twelve-month outcomes of a biolimus eluting stent with a biodegradable polymer compared with a sirolimus eluting stent with a durable polymer. EuroIntervention 2010;6(2):233–239. doi: 10.4244/eijv6i2a37
[68] Pendyala LK, Matsumoto D, Shinke T et al. Nobori stent shows less vascular inflammation and early recovery of endothelial function compared with Cypher stent. JACC Cardiovasc Interv 2012;5(4):436–444. doi: 10.1016/j.jcin.2011.11.013
[69] Kubo T, Akasaka T, Kozuma K et al. Vascular response to drug-eluting stent with biodegradable vs. durable polymer. Optical coherence tomography substudy of the NEXT. Circ J 2014;78(10):2408–2414. doi: 10.1253/circj.cj-14-0337
[70] Kuramitsu S, Iwabuchi M, Yokoi H et al. Incidence and clinical impact of stent fracture after the Nobori biolimus-eluting stent implantation. J Am Heart Assoc 2014;3(2):e000703. doi: 10.1161/jaha.113.000703
[71] Serruys PW, Farooq V, Kalesan B et al. Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimus-eluting stents in patients with coronary artery disease: final 5-year report of the LEADERS (Limus Eluted From A Durable Versus ERodable Stent Coating) randomized, noninferiority trial. JACC Cardiovasc Interv 2013;6(8):777–789. doi: 10.1016/j.jcin.2013.04.011
[72] Chevalier B, Silber S, Park SJ et al. Randomized comparison of the Nobori Biolimus A9-eluting coronary stent with the Taxus Liberte paclitaxel-eluting coronary stent in patients with stenosis in native coronary arteries: the NOBORI 1 trial–Phase 2. Circ Cardiovasc Interv 2009;2(3):188–195. doi: 10.1161/circinterventions.108.823443
[73] Chevalier B, Wijns W, Silber S et al. Five-year clinical outcome of the Nobori drug-eluting coronary stent system in the treatment of patients with coronary artery disease: final results of the NOBORI 1 trial. EuroIntervention 2015;11(5):549–554. doi: 10.4244/eijy14m12_13
[74] Natsuaki M, Kozuma K, Morimoto T et al. Biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent: a randomized, controlled, noninferiority trial. J Am Coll Cardiol 2013;62(3):181–190. doi: 10.1016/j.jacc.2013.04.045
[75] Natsuaki M, Kozuma K, Morimoto T et al. Final 3-year outcome of a randomized trial comparing second-generation drug-eluting stents using either biodegradable polymer or durable polymer: NOBORI biolimus-eluting versus XIENCE/PROMUS everolimus-eluting stent trial. Circ Cardiovasc Interv 2015;8(10):e002817. doi: 10.1161/circinterventions.115.002817
[76] Smits PC, Hofma S, Togni M et al. Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent (COMPARE II): a randomised, controlled, non-inferiority trial. Lancet 2013;381(9867):651–660. doi: 10.1016/s0140-6736(12)61852-2
[77] Cassese S, Fusaro M, Byrne RA et al. Clinical outcomes of patients treated with Nobori biolimus-eluting stent: meta-analysis of randomized trials. Int J Cardiol 2014;175(3):484–491. doi: 10.1016/j.ijcard.2014.06.014
[78] Kereiakes DJ, Meredith IT, Windecker S et al. Efficacy and safety of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent: the EVOLVE II Randomized Trial. Circ Cardiovasc Interv 2015;8(4):e002372. doi: 10.1161/circinterventions.114.002372
[79] Saito S, Valdes-Chavarri M, Richardt G et al. A randomized, prospective, intercontinental evaluation of a bioresorbable polymer sirolimus-eluting coronary stent system: the CENTURY II (Clinical Evaluation of New Terumo Drug-Eluting Coronary Stent System in the Treatment of Patients with Coronary Artery Disease) trial. Eur Heart J 2014;35(30):2021–2031. doi: 10.1093/eurheartj/ehu210
[80] Waltenberger J, Brachmann J, van der Heyden J et al. Real-world experience with a novel biodegradable polymer sirolimus-eluting stent: twelve-month results of the BIOFLOW-III registry. EuroIntervention 2016;11(10):1106–1110. doi: 10.4244/eijy15m03_08
[81] Jensen LO. Randomized comparison of a sirolimus-eluting stent with a biolimus-eluting stent in patients treated with PCI: the SORT OUT VII trial. Presented at: EuroPCR; 19 May 2015; Paris, France.
[82] Pilgrim T, Heg D, Roffi M et al. Ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable polymer everolimus-eluting stent for percutaneous coronary revascularisation (BIOSCIENCE): a randomised, single-blind, non-inferiority trial. Lancet 2014;384(9960):2111–2122. doi: 10.1016/s0140-6736(14)61038-2
[83] Desch S, Schloma D, Mobius-Winkler S et al. Randomized comparison of a polymer-free sirolimus-eluting stent versus a polymer-based paclitaxel-eluting stent in patients with diabetes mellitus: the LIPSIA Yukon trial. JACC Cardiovasc Interv 2011;4(4):452–459. doi: 10.1016/j.jcin.2010.11.016
[84] Stiermaier T, Heinz A, Schloma D et al. Five-year clinical follow-up of a randomized comparison of a polymer-free sirolimus-eluting stent versus a polymer-based paclitaxel-eluting stent in patients with diabetes mellitus (LIPSIA Yukon trial). Catheter Cardiovasc Interv 2014;83(3):418–424. doi: 10.1002/ccd.25131
[85] Costa RA, Abizaid A, Mehran R et al. Polymer-free biolimus A9-coated stents in the treatment of de novo coronary lesions: 4- and 12-month angiographic follow-up and final 5-year clinical outcomes of the prospective, multicenter BioFreedom FIM clinical trial. JACC Cardiovasc Interv 2016;9(1):51–64. doi: 10.1016/j.jcin.2015.09.008
[86] Beijk MA, Klomp M, Verouden NJ et al. Genous endothelial progenitor cell capturing stent vs. the Taxus Liberte stent in patients with de novo coronary lesions with a high-risk of coronary restenosis: a randomized, single-centre, pilot study. Eur Heart J 2010;31(9):1055–1064. doi: 10.1093/eurheartj/ehp476
[87] Vasa M, Fichtlscherer S, Adler K et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation 2001;103(24):2885–2890. doi: 10.1161/hc2401.092816
[88] Levine GN, Bates ER, Bittl JA et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 2016;134(10):e123–e155. doi: 10.1161/cir.0000000000000404
[89] Montalescot G, Sechtem U, Achenbach S et al. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34(38):2949–3003. doi: 10.1093/eurheartj/eht296
[90] Garg P, Normand SL, Silbaugh TS et al. Drug-eluting or bare-metal stenting in patients with diabetes mellitus: results from the Massachusetts Data Analysis Center Registry. Circulation 2008;118(22):2277–2285. doi: 10.1161/circulationaha.108.820159
[91] Bangalore S, Kumar S, Fusaro M et al. Outcomes with various drug eluting or bare metal stents in patients with diabetes mellitus: mixed treatment comparison analysis of 22,844 patient years of follow-up from randomised trials. BMJ 2012;345:e5170. doi: 10.1136/bmj.e5170
[92] Kim WJ, Lee SW, Park SW et al. Randomized comparison of everolimus-eluting stent versus sirolimus-eluting stent implantation for de novo coronary artery disease in patients with diabetes mellitus (ESSENCE-DIABETES): results from the ESSENCE-DIABETES trial. Circulation 2011;124(8):886–892. doi: 10.1161/circulationaha.110.015453
[93] Grube E, Chevalier B, Guagliumi G et al. The SPIRIT V diabetic study: a randomized clinical evaluation of the XIENCE V everolimus-eluting stent vs the TAXUS Liberte paclitaxel-eluting stent in diabetic patients with de novo coronary artery lesions. Am Heart J 2012;163(5):867–875.e1. doi: 10.1016/j.ahj.2012.02.006
[94] Stone GW, Kedhi E, Kereiakes DJ et al. Differential clinical responses to everolimus-eluting and paclitaxel-eluting coronary stents in patients with and without diabetes mellitus. Circulation 2011;124(8):893–900. doi: 10.1161/circulationaha.111.031070
[95] Kaul U, Bangalore S, Seth A et al. Paclitaxel-eluting versus everolimus-eluting coronary stents in diabetes. N Engl J Med 2015;373(18):1709–1719. doi: 10.1056/nejmoa1510188
[96] Vardi M, Burke DA, Bangalore S et al. Long-term efficacy and safety of zotarolimus-eluting stent in patients with diabetes mellitus: pooled 5-year results from the ENDEAVOR III and IV trials. Catheter Cardiovasc Interv 2013;82(7):1031–1038.doi: 10.1002/ccd.25045
[97] Park KW, Lee JM, Kang SH et al. Everolimus-eluting Xience v/Promus versus zotarolimus-eluting resolute stents in patients with diabetes mellitus. JACC Cardiovasc Interv 2014;7(5):471–481. doi: 10.1016/j.jcin.2013.12.201
[98] Muramatsu T, Onuma Y, van Geuns RJ et al. 1-year clinical outcomes of diabetic patients treated with everolimus-eluting bioresorbable vascular scaffolds: a pooled analysis of the ABSORB and the SPIRIT trials. JACC Cardiovasc Interv 2014;7(5):482–493. doi: 10.1016/j.jcin.2014.01.155
[99] Seth A, Hiremath S, Dani S et al. Clinical outcomes with Biolimus (A9) eluting stent, ‘BioMatrix’ in diabetic patients–interim results from multicenter post market surveillance registry in India. Indian Heart J 2013;65(5):586–592. doi: 10.1016/j.ihj.2013.08.030
[100] Iakovou I, Schmidt T, Bonizzoni E et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents.JAMA 2005;293(17):2126–2130. doi: 10.1001/jama.293.17.2126
[101] Baber U, Mehran R, Kirtane AJ et al. Prevalence and impact of high platelet reactivity in chronic kidney disease: results from the assessment of dual antiplatelet therapy with drug-eluting stents registry. Circ Cardiovasc Interv 2015;8(6):e001683. doi: 10.1161/circinterventions.115.001683
[102] Volodarskiy A, Kumar S & Bangalore S. Drug eluting vs. bare metal stents in patients with chronic kidney disease and coronary artery disease: insight from a systematic review and meta-analysis of trials. Circulation 2013;128: A16116. Available at: http://circ.ahajournals.org/content/128/Suppl_22/A16116 (accessed October 2016)
[103] Wanitschek M, Pfisterer M, Hvelplund A et al. Long-term benefits and risks of drug-eluting compared to bare-metal stents in patients with versus without chronic kidney disease. Int J Cardiol 2013;168(3):2381–2388. doi: 10.1016/j.ijcard.2013.01.257
[104] Green SM, Selzer F, Mulukutla SR et al. Comparison of bare-metal and drug-eluting stents in patients with chronic kidney disease (from the NHLBI Dynamic Registry). Am J Cardiol 2011;108(11):1658–1664. doi: 10.1016/j.amjcard.2011.07.029
[105] Baber U, Giustino G, Sartori S et al. Effect of chronic kidney disease in women undergoing percutaneous coronary intervention with drug-eluting stents: a patient-level pooled analysis of randomized controlled trials. JACC Cardiovasc Interv 2016;9(1):28–38. doi: 10.1016/j.jcin.2015.09.023
[106] Spaulding C, Henry P, Teiger E et al. Sirolimus-eluting versus uncoated stents in acute myocardial infarction. N Engl J Med 2006;355(11):1093–1104. doi: 10.1056/nejmoa062006
[107] Laarman GJ, Suttorp MJ, Dirksen MT et al. Paclitaxel-eluting versus uncoated stents in primary percutaneous coronary intervention. N Engl J Med 2006;355(11):1105–1113. doi: 10.1056/nejmoa062598
[108] Kastrati A, Dibra A, Spaulding C et al. Meta-analysis of randomized trials on drug-eluting stents vs. bare-metal stents in patients with acute myocardial infarction. Eur Heart J 2007;28(22):2706–2713. doi: 10.1093/eurheartj/ehm402
[109] Sabate M, Cequier A, Iniguez A et al. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet 2012;380(9852):1482–1490. doi: 10.1016/s0140-6736(12)61223-9
[110] Palmerini T, Biondi-Zoccai G, Della Riva D et al. Clinical outcomes with drug-eluting and bare-metal stents in patients with ST-segment elevation myocardial infarction: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol 2013;62(6):496–504. doi: 10.1016/j.jacc.2013.05.022
[111] Sarno G, Lagerqvist B, Nilsson J et al. Stent thrombosis in new-generation drug-eluting stents in patients with STEMI undergoing primary PCI: a report from SCAAR. J Am Coll Cardiol 2014;64(1):16–24. doi: 10.1016/j.jacc.2014.04.022
[112] Brugaletta S, Gori T, Low AF et al. Absorb bioresorbable vascular scaffold versus everolimus-eluting metallic stent in ST-segment elevation myocardial infarction: 1-year results of a propensity score matching comparison: the BVS-EXAMINATION Study (bioresorbable vascular scaffold-a clinical evaluation of everolimus eluting coronary stents in the treatment of patients with ST-segment elevation myocardial infarction). JACC Cardiovasc Interv 2015;8(1 Pt B):189–197. doi: 10.1016/j.jcin.2014.10.005
[113] Valgimigli M, Patialiakas A, Thury A et al. Zotarolimus-eluting versus bare-metal stents in uncertain drug-eluting stent candidates. J Am Coll Cardiol 2015;65(8):805–815. doi: 10.1016/j.jacc.2014.11.053
[114] Sabate M, Brugaletta S, Abizaid A et al. Drug eluting stent implantation in patients requiring concomitant vitamin K antagonist therapy. One-year outcome of the worldwide e-SELECT registry. Int J Cardiol 2013;168(3):2522–2527. doi: 10.1016/j.ijcard.2013.03.064
[115] Dewilde WJ, Oirbans T, Verheugt FW et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013;381(9872):1107–1115. doi: 10.1016/s0140-6736(12)62177-1
[116] Hong YJ, Jeong MH, Abizaid A et al. Sirolimus-eluting coronary stents in octogenarians: a 1-year analysis of the worldwide e-SELECT Registry. JACC Cardiovasc Interv 2011;4(9):982–991. doi: 10.1016/j.jcin.2011.06.013
[117] Mauri L, Kereiakes DJ, Yeh RW et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014;371(23):2155–2166. doi: 10.1056/nejmoa1409312
[118] Giustino G, Baber U, Sartori S et al. Duration of dual antiplatelet therapy after drug-eluting stent implantation: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Cardiol 2015;65(13):1298–1310. doi: 10.1016/j.jacc.2015.01.039
[119] Baber U, Mehran R, Giustino G et al. Coronary thrombosis and major bleeding after PCI with drug-eluting stents: risk scores from PARIS. J Am Coll Cardiol 2016;67(19):2224–2234. doi: 10.1016/j.jacc.2016.02.064
[120] Yeh RW, Secemsky EA, Kereiakes DJ et al. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA 2016;315(16):1735–1749. doi: 10.1001/jama.2016.3775
[121] Windecker S, Kolh P, Alfonso F et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35(37):2541–2619. doi: 10.1093/eurheartj/ehu278