Biophoto Associates / Science Photo Library
In this article you will learn:
- The site of action of commonly used diuretics
- How different classes of diuretics affect water reabsorption in the kidneys
- The common side effects of different diuretic classes
Diuretics are commonly prescribed medicines used to treat oedema caused by conditions such as heart failure, liver cirrhosis and kidney disease. Some diuretics may be used to treat hypertension. However, adverse effects are common and it has been estimated that diuretics contribute to around one in five of all medicines-related hospital admissions[1]
.
Diuretics work by increasing the excretion of sodium from the kidneys, drawing water along with the sodium. Around 180l of water is filtered by the kidneys each day, but only around 1.5l of this will actually leave the body.
Filtration occurs in the nephrons of the kidney, when blood passes through the glomerular capillaries at high pressure. Water and sodium are forced out of the blood and into the renal tubule at Bowman’s capsule (see ‘Diuretic sites of action’). The filtrate contains other small molecules, such as potassium and chloride ions, and glucose. Larger structures, such as red blood cells and large proteins, remain in the blood.
Reabsorption occurs as sodium and water pass through the different sections of the renal tubule. Sodium is reabsorbed via active transport and passive diffusion, whereas water is reabsorbed through osmosis, moving from areas of low electrolyte concentration to areas of high electrolyte concentration, i.e. from the renal tubule to the blood.
Anatomy and function of the nephron
Bowman’s capsule: water and electrolytes pass into the nephron from the glomerulus.
Proximal convoluted tubule: reabsorbs two thirds of sodium and water, in addition to bicarbonate and glucose.
Descending loop of Henle: passive reabsorption of water due to high osmolarity in interstitium, resulting in concentration of tubular fluid.
Thick ascending loop of Henle: reabsorbs around 20% of sodium but is impermeable to water.
Distal convoluted tubule: reabsorbs remaining sodium, water and bicarbonate; makes bicarbonate; potassium secreted into the tubule.
Collecting ducts: Fine adjustments of sodium and water; largely impermeable to water without the presence of antiduretic hormone (ADH).
Carbonic anhydrase inhibitors
Acetazolamide is the only oral carbonic anhydrase inhibitor used in practice. It decreases the amount of sodium that is reabsorbed from the proximal convoluted tubule, increasing the volume of water excreted in the urine.
Carbonic anhydrase catalyses the formation of carbonic acid from water and carbon dioxide in tubular cells. Carbonic acid then dissociates into bicarbonate ions (which are reabsorbed into the blood) and hydrogen ions, which are exchanged for sodium ions into the lumen of the tubule. Reversible inhibition of carbonic anhydrase results in reduced sodium and hydrogen ion exchange and thus increases excretion of sodium, bicarbonate and water.
Acetazolamide is considered a weak diuretic because excess sodium delivered from the proximal tubule can be reabsorbed further along the renal tubule[2]
.
The loss of bicarbonate in the kidney can cause metabolic acidosis, which can be useful when acetazolamide is used in combination with a loop diuretic to prevent metabolic alkalosis (see below).
Acetazolamide is not commonly used as a diuretic, and is mainly prescribed for glaucoma to reduce intraocular pressure by suppressing the secretion of bicarbonate and water into the aqueous humour[3]
. It is also used unlicensed as prophylaxis for altitude sickness.
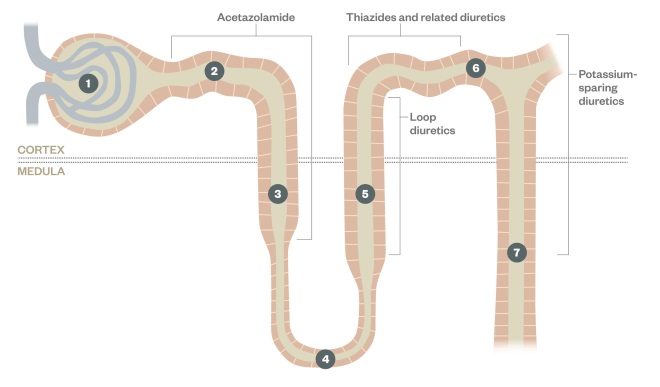
Diuretic sites of action
The different classes of diuretic have distinct sites of action which determines their effectiveness and side effect profile
1: Bowman’s capsule and glomerulus
2: Proximal convoluted tube
3: Thick descending limb of the Loop of Henle
4: Loop of Henle
5: Thick ascending limb of the Loop of Henle
6: Distal convoluted tubule
7: Collecting duct
Loop diuretics
The main site of sodium reabsorption is in the thick ascending limb of the loop of Henle. Here, sodium ions are actively transported out of the tubule by the membrane transport protein sodium-potassium-chloride (Na+/K+/2Cl–) cotransporter. Loop diuretics (e.g. furosemide, bumetanide and torasemide) compete with chloride ions on this transporter to inhibit sodium, potassium and chloride reabsorption.
In normal circumstances, the reabsorption of sodium at this location creates a hypertonic gradient, which causes water reabsorption via osmosis from the descending loop of Henle. Loop diuretics reduce sodium reabsorption, thereby reducing the hypertonicity in the interstitium of the medulla and leading to reduced reabsorption (and therefore increased excretion) of water.
As up to 20% of the filtered sodium is reabsorbed in the loop of Henle, loop diuretics can exert a much greater effect on sodium reabsorption than any other diuretic acting elsewhere in the nephron[4]
, and cause the most profuse diuresis.
Loop diuretics must reach a certain concentration in the tubular lumen to have an effect. Further dose increases will then continue to produce additional diuresis until a maximum ‘ceiling’ concentration has been reached. It is believed that cotransporter mechanisms become saturated at this point[5]
, and increasing the dose further will not offer any additional benefits but can increase the risk of side effects.
Furosemide and bumetanide both act within one hour of oral administration and stop having an effect after around six hours, after which rebound reabsorption of sodium further in the tubule may occur. This is known as a “braking” effect, and is a self-protection mechanism by the kidneys, activated to prevent dehydration.
Rebound sodium reabsorption can be prevented by increasing the frequency of diuretic administration or reducing sodium intake[5]
.
Hyponatraemia is a common side effect of loop diuretics and is caused by increases in sodium excretion. In normal circumstances, some of the potassium that is reabsorbed by the Na+/K+/2Cl– cotransporter is recycled back into the tubular fluid, which drives further sodium, calcium and magnesium reabsorption. Loop diuretics inhibit potassium recycling, resulting in reduced calcium and magnesium reabsorption[4]
.
Loop diuretics also have the potential to cause hypokalaemia and metabolic alkalosis. This is because the inhibition of sodium reabsorption at the loop of Henle results in increased sodium reabsorption further along the kidney tubule (in the late distal tubule and collecting duct), at the expense of hydrogen and potassium reabsorption[6]
. Loop diuretics can also cause distal compensation: increased reabsorption of sodium in the distal convoluted tubule.
High doses of loop diuretics may lead to transient or permanent deafness, which may be caused by the diuretic binding to an isozyme of the Na+/K+/2Cl– cotransporter in the inner ear. The risk of the side effect can be minimised by infusing intravenous preparations slowly (e.g. 4mg/min maximum for furosemide), avoiding concomitant use with other medicines that can cause toxicity in the ear and avoiding prolonged use where possible.
Loop diuretics are mainly prescribed for oedema and are not generally used to manage hypertension unless fluid overload is a contributing factor. When using diuretics to reduce fluid load, the patient’s weight and fluid balance should be monitored regularly. Weight loss should be no more than 0.5–1kg per day, which is equivalent to a negative fluid balance of 500–1,000ml per day: any greater and the patient is at increased risk of hypovolaemia, hypotension and acute kidney injury.
Bumetanide is the loop diuretic of choice for patients with significant gut oedema. This is because its absorption profile is more predictable than that of furosemide.
Thiazides and related diuretics
Thiazides and related diuretics inhibit the Na+/Cl– cotransporter in the distal tubule, preventing sodium reabsorption. Thiazides and related diuretics inhibit approximately 3% to 8% of sodium reabsorption, and so exert a much smaller diuretic effect than loop diuretics[2]
. They are therefore considered ‘low ceiling’ diuretics. However, they are potent and small doses can have profound effects.
Thiazides and related diuretics are transported into the tubule in exchange for uric acid. This can lead to raised serum uric acid levels and may precipitate gout. Continuation of thiazide therapy in patients who develop gout should be considered on an individual basis.
The absorption of magnesium in the distal convoluted tubule occurs through a magnesium channel that is exclusively expressed in this location. Thiazides and related diuretics can down-regulate the expression of this transport protein, causing hypomagnesaemia[7]
.
Hypercalcaemia is another known adverse effect of thiazide and related diuretics. The mechanism is not well understood, but it is thought that the contraction of extracellular volume that occurs after inhibition of sodium and water reabsorption at the distal convoluted tubule leads to a compensatory increase in sodium and water reabsorption at the proximal convoluted tubule, and drives the passive reabsorption of calcium[8]
.
Thiazides and related diuretics may also cause hypokalaemia and metabolic alkalosis via a similar mechanism to loop diuretics.
Thiazides and related diuretics are commonly used for the treatment of hypertension[9]
. This is not due to their direct diuretic effect, but because they cause vasodilation (the mechanism for this is poorly understood). They are no longer recommended by the National Institute for Health and Care Excellence as first-line treatment options for hypertension as they may cause metabolic complications such as hyperglycaemia, lipid abnormalities and other electrolyte abnormalities. The thiazide-like diuretics chlortalidone and indapamide are thought to have lower incidence of these effects than bendroflumethiazide and hydrochlorothiazide, and are therefore the treatment of choice if a thiazide or related diuretic is required.
Thiazides and related diuretics can become relatively ineffective in patients with severe chronic kidney disease (stages 4 and 5) if they are not prescribed with a loop diuretic[10]
. They can also be highly effective if used in combination with loop diuretics to treat resistant oedema. The medicines most commonly used in combination with loop diuretics are metolazone and bendroflumethiazide. Metolazone is effective for patients with a glomerular filtration rate of <20ml/min. It is believed to have a secondary site of action in the proximal convoluted tubule[11]
.
Potassium-sparing diuretics
Potassium-sparing diuretics can be divided into two groups: sodium channel blockers (triamterene and amiloride) and aldosterone antagonists (spironolactone and eplerenone). They act in the late distal tubule and the collecting duct, where just 2–3% of sodium reabsorption occurs.
Normally, cells in the collecting duct retain sodium by reabsorbing sodium ions through sodium channels, which is then exchanged with potassium from the interstitial fluid due to the action of the enzyme Na+/K+ adenosine triphosphatase (Na+/K+ ATPase). The potassium drawn in to the cell is then excreted into the lumen by potassium channels and lost in urine.
Sodium channel blockers inhibit the sodium channels of principal cells. This prevents sodium reabsorption and reduces sodium levels in the cell. This in turn reduces the activity of the Na+/K+ ATPase, meaning less potassium to be pumped into the cell from the interstitial space and subsequently lost from the body[6]
.
Amiloride and triamterene may be used to prevent potassium loss when other classes of diuretics are being used, and for conditions that cause potassium wasting, e.g. Gitelman syndrome.
Aldosterone is a hormone that causes principal cells, which are located in the collecting duct, to increase sodium and water reabsorption by increasing the number of active sodium channels and the activity of Na+/K+ ATPase. It also causes loss of potassium and hydrogen ions by increasing the number of active potassium channels and activity of the enzyme H+ ATPase.
Spironolactone and eplerenone bind to the aldosterone receptor and reduce its effects, leading to sodium and water excretion and reduced potassium excretion[6]
.
Spironolactone is used to treat secondary hyperaldosteronism, the excess secretion of aldosterone that can occur in patients with cardiac failure or liver failure.
Spironolactone is the first-line diuretic for the treatment of ascites[12]
and is indicated as an adjunct therapy for severe chronic heart failure (New York Heart Association stages 3 and 4)[13]
.
Eplerenone has been licensed for acute heart failure following myocardial infarction[14]
. It causes minimal anti-androgenic side effects (e.g. decreased libido, impotence, gynaecomastia in men and menstrual irregularity in women) and could be used in preference to spironolactone for patients who experience these adverse effects[15]
.
Metabolic acidosis can occur with both classes of potassium-sparing diuretics. Potassium-sparing diuretics may be used with thiazide and related or loop diuretics to counteract metabolic alkalosis.
Potassium-sparing diuretics can cause hyperkalaemia, and potassium levels should be monitored regularly if they are used in patients with renal disease or who are taking an ACE inhibitor, angiotensin-II receptor antagonist, or potassium supplements.
Potassium-sparing diuretics should be used with caution in patients with renal disease and potassium levels should be monitored regularly for all patients.
Diuretic resistance
Diuretic resistance can occur in heart failure, liver cirrhosis and kidney disease through a variety of mechanisms. Reduced blood flow to the kidney and competition with accumulating endogenous anions (such as urea, urate and bile acids) for transporters impairs diuretic secretion in the proximal tubule[16]
. In heart failure and liver cirrhosis, increased aldosterone increases sodium absorption in the collecting tubule[17],[18]
.
Common ways to overcome resistance include sodium restriction, increasing the dose and frequency of the diuretic, using intravenous furosemide and combining a loop diuretic with a thiazide to counteract distal compensation.
About the authors
Kathrine Parker MPharm, MRPharmS, NMP,
Marc Vincent BPharm, MRPharmS, NMP,
Mustafa Mohammed, MPharm,
Jalak Shah MPharm are pharmacists at Central Manchester Hospitals NHS Foundation Trust.
This article is the edited result of a team-based learning exercise on diuretics.
References
[1] Pirmohamed M, James S, Meakin S et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18,820 patients. BMJ 2004;329:15–19.
[2] Rose BD. Diuretics. Kidney Int 1991; 39(2):336.
[3] Neal MJ. Medical pharmacology at a glance. Third edition. Oxford: Blackwell; 1997.
[4] Battista E, Horton-Szar D & Page C. Crash course: Pharmacology. Fourth Edition. London: Mosby Elsevier; 2012.
[5] Brater DC. Diuretic Therapy. N Engl J Med 1998;339(6):387–395.
[6] Page CP, Curtis MJ, Sutter MC et al. Integrated Pharmacology. London: Mosby; 1997.
[7] Xi Q, Hoenderop GJ & Blindel S. Regulation of magnesium reabsorption in DCT. Pflugers Archiv Eur J Physio 2009;458: 89–98.
[8] Nijenhius T, Vallon V, Van Der Kep AWCM et al. Enhanced passive calcium reabsorption and reduced magnesium channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. Journ Clin Invest 2005;115:1651–1658.
[9] National Institute for Health and Care Excellence. Hypertension. London:NICE 2011.
[10] Fliser D. Loop diuretics and thiazides: the case for their combination in chronic renal failure. Nephrol Dial Transplant 1996;11:408–423.
[11] Wilcox CS. New insights into diuretic use in patients with chronic renal disease. J Am Soc Nephrol 2002;13:798–805.
[12] Moore KP & Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut 2006;55:1–12.
[13] National Institute for Health and Care Excellence. Chronic heart failure: Management of chronic heart failure in adults in primary and secondary care. London:NICE 2010.
[14] Pitt B, Remme W, Zannad F et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309–1321.
[15] Struthers A, Krum H & Williams GH. A Comparison of the Aldosterone-blocking agents Eplerenone and Spironolactone. Clin Cardiol. 2008; 31(4): 153–158.
[16] Voelker JR, Cartwright-Brown DC, Anderson S et al. Comparison of loop diuretics in patients with chronic renal insufficiency. Kidney Int 1987;32:572–578.
[17] De Bruyne LKM. Mechanisms and management of diuretic resistance in congestive heart failure. Postgraduate Medical Journal 2003;79:268–271.
[18] Hropot M, Fowler N, Karlmark B et al. Tubular actions of diuretics: distal effects on electrolyte transport and acidification. Kidney Int 1985;28(3):477.
You might also be interested in…
Dialysis: principles and treatment options
Dialysis: management