SAM OGDEN / SCIENCE PHOTO LIBRARY
After reading this article, you should be able to:
- Identify the risk factors for developing hepatocellular cancer (HCC) and understand which patients require surveillance;
- Know how HCC is diagnosed;
- Understand the main treatment options for HCC and which patients are eligible for curative treatment.
Introduction
Hepatocellular carcinoma (HCC) is the most common form of primary liver cancer and is a growing global health concern. It is the sixth most common cancer worldwide, with 905,700 new cases in 2020, and is the fourth most common cause of cancer-related death, responsible for 830,200 deaths each year[1,2].
The incidence of HCC is rising: new cases have increased by 160% over the past three decades in the UK alone, with around 17 new cases diagnosed per day[3,4]. The average age at diagnosis is 69 years and the majority of cases occur in men[4]. Prognosis remains poor, with five-year survival rates less than 20%; this is unchanged in the past decade despite advances in therapeutics[5]. Considering the rise in cases and the potential burden of the condition for patients, it is important that pharmacists are aware of the signs of HCC and know how to appropriately refer.
This article will outline the importance of surveillance and how HCC is diagnosed, and will provide an overview of the current available treatment options, including the involvement of pharmacy teams where appropriate.
Cancer learning ‘hub’
Pharmacists are playing an increasingly important role in supporting patients with cancer, working within multidisciplinary teams and improving outcomes.
However, in a rapidly evolving field with numbers of new cancer medicines is increasing and the potential for adverse effects, it is now more important than ever for pharmacists to have a solid understanding of the principles of cancer biology, its diagnosis and approaches to treatment and prevention.
This new collection of cancer content, brought to you in partnership with BeOne Medicines, provides access to educational resources that support professional development for improved patient
Epidemiology
The epidemiology of HCC varies by region. Globally, viral hepatitis remains the most common cause of HCC[6]. Hepatitis B virus (HBV) is directly oncogenic and is the most significant risk factor for developing HCC — it is responsible for 50% of HCC cases[6]. HBV is the main causative factor of HCC in East Asia, which has the highest HCC incidence in the world[6]. Hepatitis C virus (HCV) remains the most common aetiology of HCC in Western Europe, North America and Japan[7]. However, the attributable risk of HCV has decreased since the advent of antiviral medication, which results in sustained virological response (undetectable viral load six months after completion of treatment)[8].
In the UK and United States, alcohol-related liver cirrhosis and non-alcoholic steatohepatitis (NASH) are significant causative factors for HCC. The incidence of NASH is rising as metabolic syndrome becomes a common phenomenon and 20% of HCC cases have NASH as the attributable factor in the UK and United States[9].
Increasing age, male sex and smoking are also independent risk factors for HCC[1,10]. Other, rarer aetiologies include alpha 1 antitrypsin disorder, haemochromatosis and primary biliary cholangitis[3].
Surveillance
Owing to poor prognosis and low surveillance uptake, HCC presents unique challenges to the NHS because half of UK cases are considered preventable[11]. Around 90% of HCC cases have a background of cirrhosis or chronic liver disease, identifying individuals with these conditions as target ‘at risk’ populations for receipt of a surveillance test. In HCC, potential benefits of early detection are that curative therapies, such as resection or transplantation, could be offered sooner, resulting in improved survival rates[12].
Biannual ultrasound and alpha-fetoprotein (AFP) measurement are the diagnostic tests used for surveillance; they are widely available, affordable and have evidence supporting their utility. Research involving cohort studies and mathematical modelling conclude that HCC surveillance results in earlier diagnosis, receipt of curative treatments and improved survival[12]. Results from several studies have showed that HCC surveillance is cost-effective, although there is ongoing debate as to whether this is consistent across all aetiologies of CLD[13,14]. While recommendations regarding inclusion of AFP as part of HCC surveillance varies between guidelines, there is consensus among all professional bodies that ultrasound is required for HCC surveillance[15–17]. In the UK, biannual ultrasound is recommended, following European Association for the Study of the Liver guidelines (see Table 1[15–17]).
There are significant challenges and controversies regarding HCC surveillance. Implementation and uptake of surveillance is suboptimal, with rates of surveillance uptake of less than 20% in the United States[18].
Low uptake is compounded by poor ultrasound sensitivity, which is 63% for lesions <2cm[19]. The harms of surveillance relate to false positive or indeterminate results and subsequent tests, including further imaging, which confers radiation risks, or biopsy, which is highly invasive. In one study, physical harm (defined as any unnecessary test or procedure arising from a false positive or indeterminate ultrasounds) was experienced by 22.8% of patients[20]. While most of these patients were classified as experiencing ‘mild’ or ‘moderate’ harm from repeated imaging, a small number underwent more invasive investigation including biopsy or angiography.
Diagnosis
Clinical presentation
The clinical presentation of HCC can vary greatly. Patients with early-stage HCC are frequently asymptomatic, with radiological diagnosis made during image surveillance. In late-stage disease, given the majority of HCC occurs in chronic liver disease, patients often present with evidence of decompensated liver disease, including jaundice, encephalopathy, ascites and variceal bleeding. Patients may also experience right upper quadrant pain and weight loss, in particular with evidence of sarcopenia (i.e. loss of muscle mass)[3].
Radiological diagnosis
Radiology is the backbone of HCC diagnosis, exploiting the vascular properties of the liver. The liver receives dual blood supply from the portal vein and hepatic artery, paired with the bile duct as part of the portal triad (see Figure 1[21]). During carcinogenesis there is increased oxygen requirement as precancerous dysplastic nodules progress to HCC, leading to secretion of angiogenic growth factors, such as vascular endothelial growth factor (VEGF)[22,23]. This results in new unpaired arteries supplying HCC tissue, with progressive loss of portal venous supply (see Figure 2[24]).
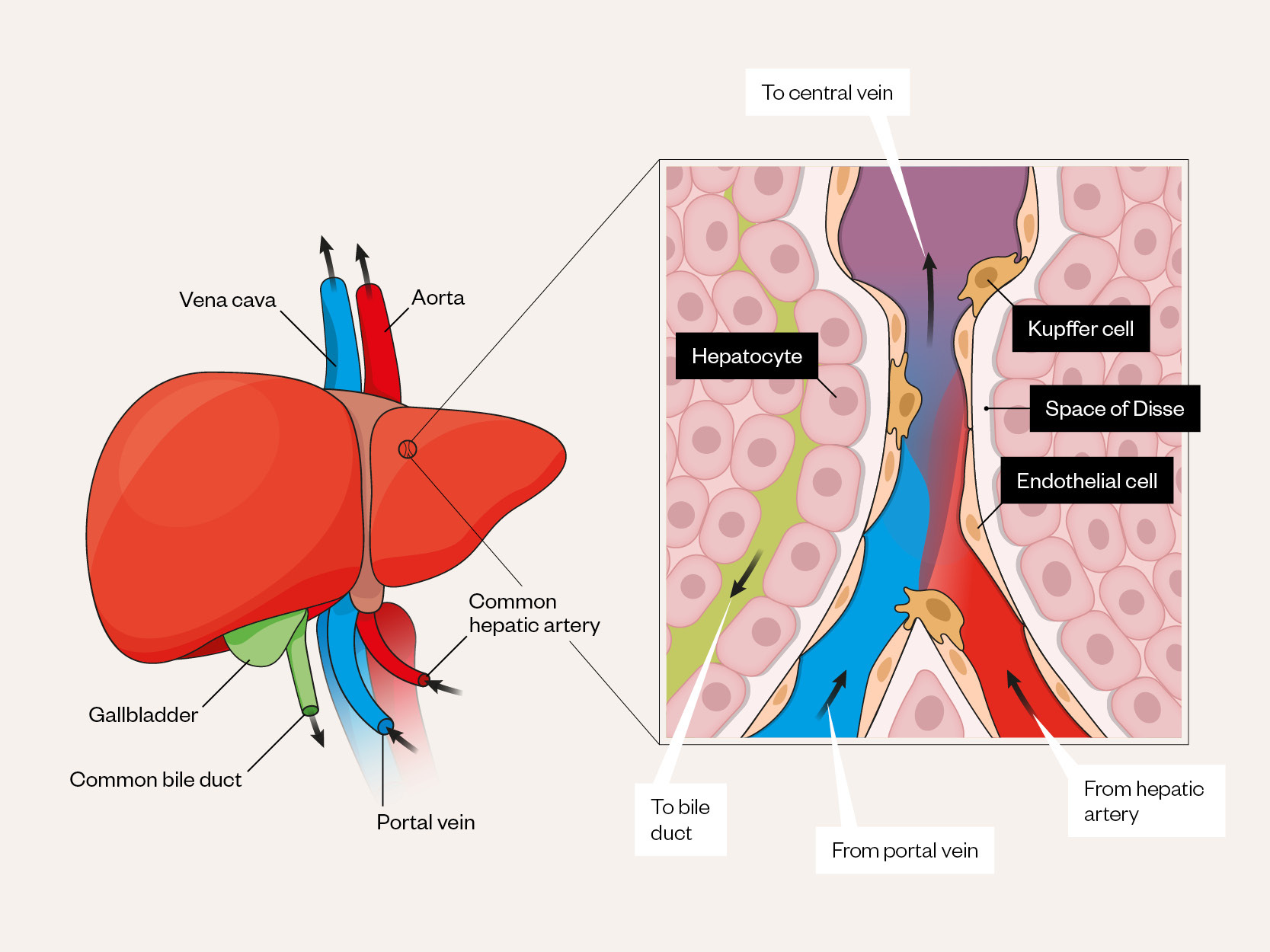
Adapted and reused with permission from Nakhleh
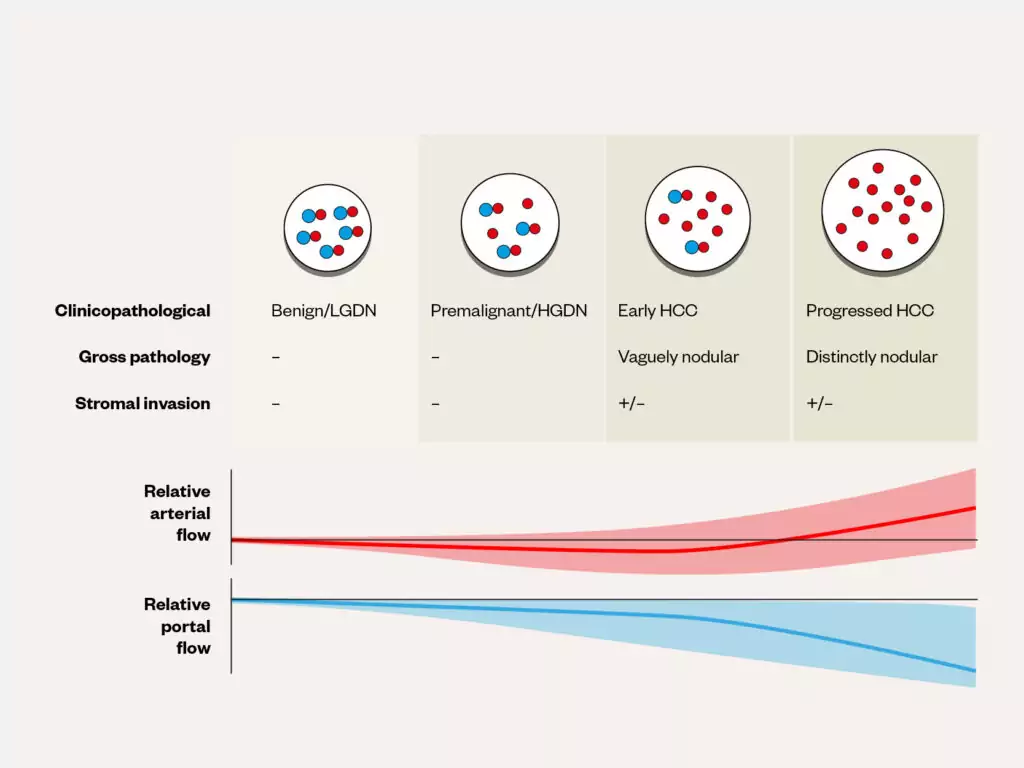
Adapted and reused with permission from Fung et al
Dynamic contrast-enhanced imaging
The differentiation in tumour and background liver blood supply can be utilised using dynamic contrast-enhanced imaging, with CT and MRI, the gold standard imaging modalities for HCC diagnosis[15,16]. Although ultrasound is favourable for HCC surveillance, it has a low sensitivity and positive predictive value compared with contrast-enhanced imaging[25]. In some expert centres, contrast-enhanced ultrasound (CEUS) can be employed for HCC diagnosis, especially in cases of inconclusive CT/MRI[26]. However, given the lack of high-quality prospective studies assessing CEUS and its inferior sensitivity for smaller HCC lesions, it is not the recommended first-line imaging modality for confirming diagnosis[15,16].
The hallmark radiological features of HCC are hyperenhancement during the hepatic arterial phase, followed by washout during the portal venous and delayed phases (see Figure 3[27]). For dynamic contrast-enhanced imaging, CT and MRI utilise iodine-based and gadolinium-based agents, respectively. Traditional extracellular gadolinium-based agents are exclusively renally excreted and not absorbed by hepatocytes, highlighting HCC arterial hyperenhancement and portal venous washout with liver parenchyma hyperenhancement, like CT imaging. However, hepatobiliary agents, such as godoxetic acid, are absorbed intracellularly by functioning hepatocytes, leading to enhancement of liver parenchyma. These hepatobiliary agents allow for further differentiation of malignant tissue from background liver during the hepatobiliary phase, 20 minutes after contrast injection (see Figure 4[28]), compared with extracellular gadolinium-based agents. Inclusion of hepatobiliary phase imaging has been shown to improve the detection of early and atypical HCC lesions compared with contrast-enhanced CT and conventional MRI[25,29,30].
The ‘Liver Imaging Report and Data System’ (LI-RADS) diagnostic algorithm has been developed to determine the probability that a given lesion is HCC[31]. Table 2 and Table 3 show the major and ancillary CT/MRI imaging features in the LI-RADS algorithm.
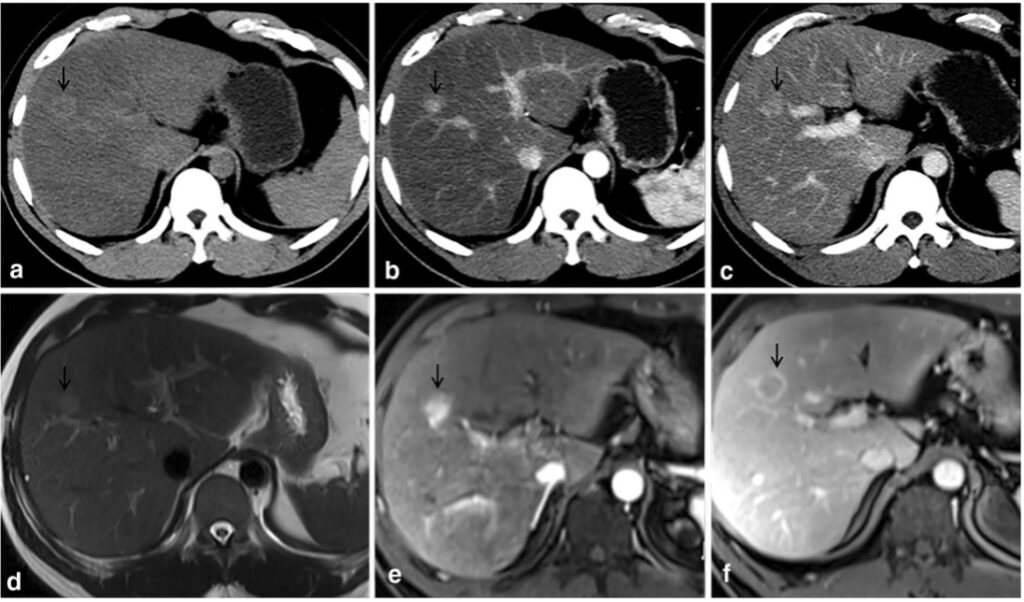
The black arrow denotes HCC lesion on pre-contrast CT (a) and MRI (d). Lesion hyperenhancement seen on late arterial phases on CT (b) and T2-weighted MRI (e). Washout on portal venous phase imaging on CT (c) and MRI (f)
Adapted and reused with permission from Tang M, Li Y, Lin Z, et al
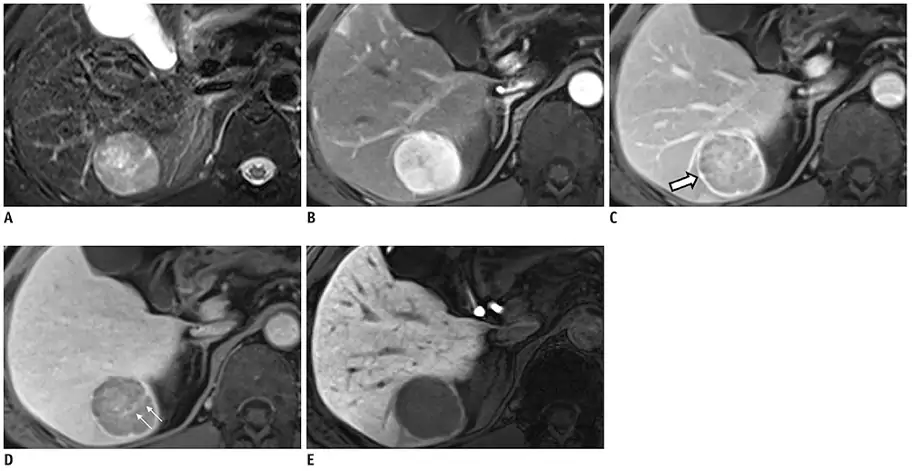
Changes in the lesion (see white arrows) and background liver across distinct phases allows for diagnosis of HCC. Pre-contrast T2 weighted appearances of HCC seen in (A). Arterial hyperenhancement seen in late arterial phase (B). Washout and capsule appearance (white arrow) seen during portal venous phase (C). Internal septum seen on transitional phase (D). Hypoenhancement compared to enhanced liver parenchyma on hepatobiliary phase (E)
Adapted and reused with permission from Kim JH, Joo I, Lee JM
Histological diagnosis
Owing to its distinct radiological features, HCC is the only solid organ tumour that does not mandate tissue confirmation for diagnosis[17,32,33]. Lesions displaying important hallmarks of HCC on imaging (i.e. arterial phase hyperenhancement, non-peripheral washout and capsule enhancement) is sufficient for non-invasive diagnosis. Pathological confirmation with liver biopsy is recommended in cases of non-cirrhotic HCC or where the diagnosis of HCC remains uncertain[15–17]. There is a perceived risk of complications caused by liver tumour biopsies, including bleeding and tumour seeding. Although tumour seeding rates vary between 1.8% to 4.0%, there is no reported evidence of an impact on patient survival[34]. As such, liver biopsy should not be precluded in cases of diagnostic uncertainty and should be encouraged to improve further molecular and genomic understanding of HCC[35].
Serum biomarkers
AFP is the only biomarker to fulfil the standardised biomarker validation[36,37]. AFP has been used as a biomarker in HCC surveillance. Although AFP shows a high specificity for HCC, its sensitivity is poor, missing more than half of early and small HCCs[38].
The addition of AFP to ultrasound for surveillance improves sensitivity for early HCC detection compared with ultrasound alone, but at the cost of a lower specificity[39]. As such, the use of AFP in surveillance and diagnosis of HCC is not actively endorsed in current practice, although it is still adopted in some centres[15,16]. AFP has a high utility in predicting survival and cancer recurrence post liver transplant[40,41]. Elevated AFP can therefore be used as selection criteria for liver transplant[41–43]. Other biomarkers, such as AFL-L3, an isoform of alpha-fetoprotein, and des-gamma-carboxy prothrombin (DCP), have been proposed in liver surveillance and prognostication but lack sufficient validation for clinical use[44].
Staging and treatment strategies
Once HCC is diagnosed, as with other cancers, prognostic assessment and classification is required to enable selection of appropriate treatment options. In most patients with HCC, the coexistence of cancer and liver cirrhosis can complicate both the assessment of prognosis and the selection of treatments[15]. One of the most commonly used staging systems is the Barcelona-Clinic Liver Cancer (BCLC) staging system, which incorporates both prognostic and treatment dependent variables and has incorporated advances in management including the newer systemic therapies and ablation for very early cancers[15,45]. The modified BCLC staging system and treatment strategy, which is endorsed by the European Association for the Study of the Liver, is shown in Figure 5[45].
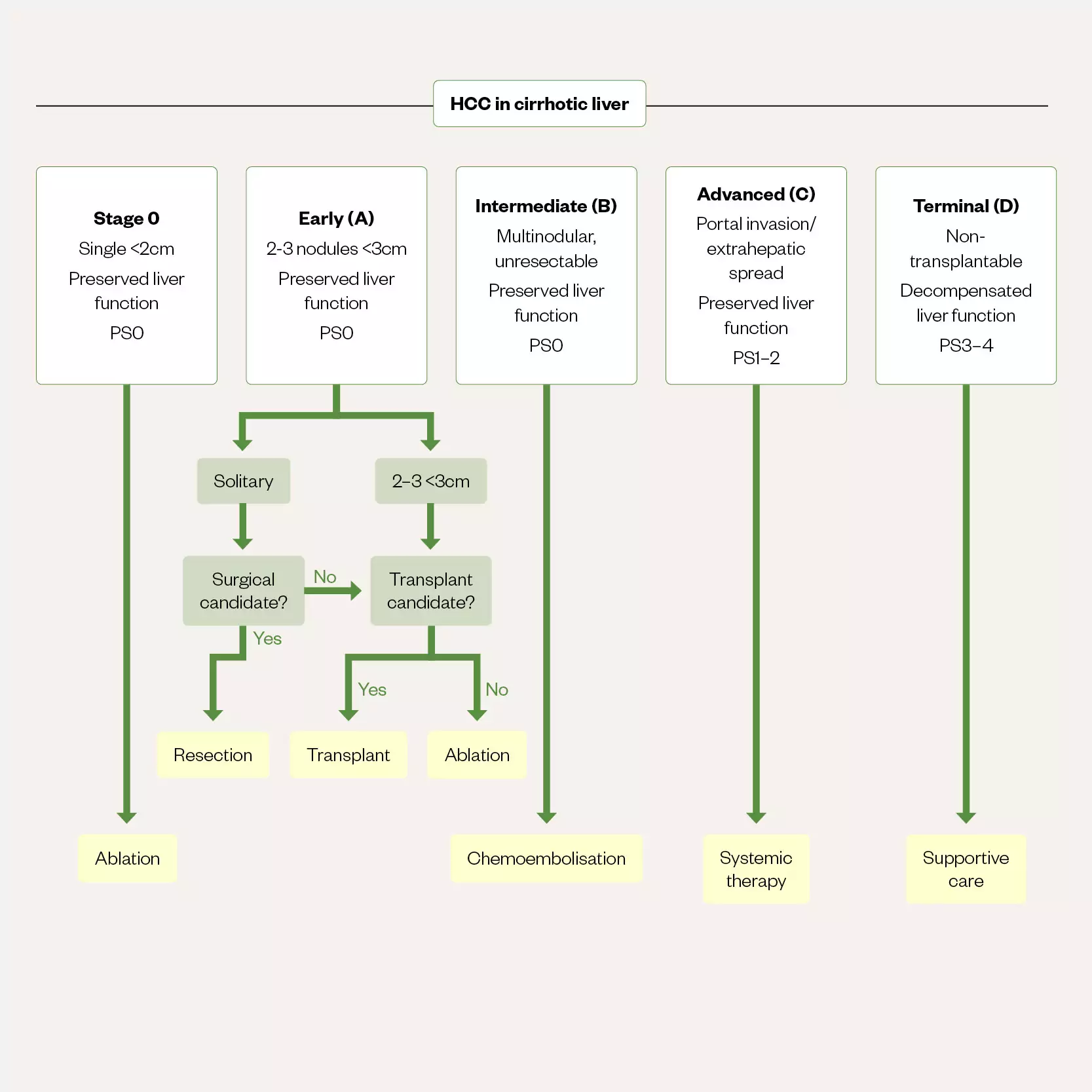
Early and very early stage (0/A) cancers with preserved liver function (that is Childs-Pugh A without any ascites) and performance status (PS) 0 can be considered for ablation, transplant or resection. Optimal surgical candidates are selected based upon several factors including CPA, degree of portal hypertension and an acceptable amount of remaining liver tissue, as well as the safety of any surgical approach. In those patients with intermediate (stage B) disease chemoembolisation is the best option and those with advanced disease (stage C) are best served with systemic therapies). Stage D, unless transplantable, does not have any treatment options beyond best supportive care
Resection
Resection of solitary tumours has good results with 80–90% five-year survival; it remains the optimal treatment for larger solitary lesions or more peripheral lesions that are laparoscopically accessible and not amenable to interventional radiology treatments as targeting their blood supply is more technically challenging[46,47].
In patients with non-cirrhotic HCC, complication rates can be low[48]. Resection of HCC in NAFLD/NASH is associated with increased postoperative complications, although mortality is relatively low and long-term survival may be higher than in resections performed in the context of chronic viral hepatitis[49].
In cirrhotic livers there are three main limitations to consider for resection. The first is the functional status of the liver and whether enough parenchyma will remain postoperatively to enable good liver function. The second is the bleeding risk conferred by the presence of clinically relevant portal hypertension (CRPH). The third is the extent of the hepatectomy required and the degree of surgical invasiveness (open versus laparoscopic)[15].
These factors contribute to the risk of hepatic decompensation post-operatively and require individualised risk assessment. Multiparametric models incorporating the presence of portal hypertension, MELD score and extent of hepatectomy can predict both the likelihood of decompensation and liver-related mortality[50]. Consensus guidelines are that the ideal patient has a solitary tumour and preserved liver function in the absence of CRPH[15]. However, improvements in pre-, intra- and peri-operative care have led to experienced centres carrying out procedures in carefully selected patients that do not fulfil these criteria with good results[15].
Transplant
The ‘Milan Criteria’ was developed in 1996 and defined a maximum criteria for liver transplant of one tumour less than 5cm or three tumours less than 3cm, and no evidence of microvascular invasion[51]. A small prospective study of 48 patients demonstrated that survival at one and five years was 84% and 74%, respectively, with a recurrence rate of 8%, and in the subgroup of patients that were shown definitively to be within Milan Criteria had four-year survival of 95%. The criteria from this small single-centre study have since been validated in larger cohorts with similar outcomes for survival[52–54].
Locoregional therapies
Ablation
Aside from transplantation and resection, radiofrequency or microwave ablation is the third potentially curative option for HCC[15,33,55]. Unlike resection, ablation can be undertaken in patients with moderately decompensated liver disease as the risk of provoking further decompensation appears lower[33,56]. Ablation is recognised as providing equivalent survival at a lower cost than resection for small lesions <2cm; above 2cm recurrence rates are higher[57–61].
Guidelines suggest that ablation should be considered for small lesions, usually those less than 3cm, including multiple small, discrete lesions — traditionally three or less — or larger lesions up to 5cm on occasion[15,33,55]. It can be challenging to obtain a sufficient treatment margin for larger lesions and it is recognised that such treatment is unlikely to be curative owing to high rates of local recurrence. This may be owing to marginal microsatellites that are not ablated in large tumours with smaller margins, and these can cause early recurrence[62,63].
As well as radiofrequency and microwave ablation, there are other ablation techniques being investigated, including cryoablation, irreversible electroporation and laser ablation, but these are not yet in widespread use[64].
Transarterial chemoembolisation, transarterial radioembolisation and stereotactic body radiotherapy
Patients with preserved liver function and multi-nodular disease, those not suitable for ablation or resection for technical reasons (for example, size, difficulty accessing the tumour, or close proximity of the tumour to adjacent structures such as the vasculature or the gall bladder which may be damaged during an ablation), can be considered for alternative locoregional therapies[15,33,55]. Unlike ablation, these alternative therapies are rarely curative. These treatments are not suitable for those with more advanced cirrhosis — for example, those with a Child-Pugh score ≥B8 — as these treatments can provoke hepatic decompensation negating the benefit from reduced tumour burden[15,29,36,55,64,65].
The most widely used locoregional therapy, aside from ablation, is transarterial chemoembolisation (TACE). In conventional TACE, a lipiodol-based emulsion is prepared with an embolising agent — for example, a contrast agent — and suffused with a cytotoxic drug, most commonly doxorubicin, cisplatin or irinotecan. This preparation is then selectively injected into the arterial blood vessel supplying the tumour during angiography; exploiting the dual blood supply of the liver and targets the tumour while minimising collateral damage to the healthy liver tissue. The liver parenchyma receives blood from both the hepatic artery and portal vein; however, HCC are almost entirely supplied from the hepatic artery, hence arterial (chemo)embolisation will predominantly affect cancer tissue sparing healthy tissue with dual supply from the portal vein. Cancer tissue is degraded by both the loss of its blood supply, and by the local delivery of the cytotoxic agent. Specialist pharmacists are integral to this process, as the success of TACE relies on an efficacious chemo-embolic agent balancing effective embolisation with drug delivery[66].
An alternative approach to TACE is to perform bland embolisation (transarterial embolisation [TAE]), which involves embolising the tumour’s arterial blood supply without using a cytotoxic agent. TAE aims to treat the target lesion purely by occluding its blood supply. Other alternative techniques include drug-eluting bead TACE (DEB-TACE), which uses microspheres carrying the cytotoxic agent to effect sustained local drug delivery, and transarterial radioembolisation (TARE) using a radioactive embolising agent instead of a conventional cytotoxic agent[66].
Stereotactic body radiotherapy (SBRT) is an emerging technique that can deliver focused, high-dose radiotherapy to tumours[64]. The role of SBRT is not yet established but early evidence suggests it might offer comparable local control to radiofrequency ablation, although lower survival has been seen[55,67,68]. SBRT appears unlikely to displace ablation as the first-line curative treatment for small tumours but is likely to have a role alongside TACE in more advanced disease. For example, SBRT can be safely used in patients with portal vein thrombosis, or to treat tumours adjacent to the biliary system, unlike TACE[69,70].
Locoregional therapies, including TACE and radiofrequency ablation, increasingly allow tumours to be ‘downstaged’ such that they meet Milan criteria for transplantation[71,72]. It is likely that this role will expand in the future, as transplant criteria evolve[15,33,55].
Systemic therapy
Patients with diffuse, infiltrative liver disease, extrahepatic spread, or portal vein invasion (BCLC-C) are not suitable for surgery or locoregional therapy and should be considered for systemic therapy[15,33,55]. For many years, sorafenib, an oral multiple tyrosine kinase inhibitor (TKI), was the sole agent approved for use in HCC — offering a median overall survival of around 10 months compared with 8 months in untreated patients[15]. However, the combination of atezolizumab, a monoclonal antibody against programmed cell death-ligand 1 (PD-L1), and bevacizumab, a monoclonal antibody that inhibits VEGF A, has recently been shown to be superior to sorafenib with a median overall survival of 19 months — this combination, known as ‘atezo-bev’, is currently the preferred regimen[55,73,74]. Durvalumab, a monoclonal antibody against PD-L1, after a priming dose of tremelimumab, a monoclonal antibody against cytotoxic T-lymphocyte-associated protein 4, may also be superior to sorafenib[75]. Hence immunotherapy is now considered the first line systemic therapy for HCC[55].
Patients considered for systemic therapy must have relatively preserved liver function, usually adjudged by the widely used Childs-Pugh score. Atezo-bev is usually restricted to those with Childs-Pugh A cirrhosis, although sorafenib was historically used in selected patients with Childs-Pugh B cirrhosis — with significantly reduced survival[15,23,55,76,77]. Bevacizumab is associated with an increased bleeding risk; therefore, there is a low threshold for endoscopy screening in patients with evidence of portal hypertension[78]. Patients with clinically significant varices despite use of a non-selective beta-blocker or a programme of variceal band ligation should not commence atezo-bev[79,80]. Anticoagulant use, vascular (for example, recent stroke or myocardial infarction) and autoimmune disorders (for example, active ulcerative colitis), hypertension, and prior liver transplantation may all preclude treatment with atezo-bev[79].
Sorafenib is used if atezo-bev is ineffective or not tolerated, or first line if atezo-bev is contraindicated. If patients tolerate but progress on sorafenib, regorafenib (an oral multi-kinase inhibitor) is indicated as second- or third-line therapy as it may extend survival[81]. Cabozantinib, a small-molecule TKI, offers an additional second- or third-line option, irrespective of a patient’s tolerance of sorafenib or prior use of a second-line option such as regorafenib[82]. Ramucirumab, an anti-angiogenic VEGF receptor-2 monoclonal antibody, has been shown to have survival benefit for those with an AFP >400ng/mL[83].
Lenvatinib, a multi-kinase inhibitor, is an alternative to sorafenib and is used in a similar manner (e.g. if atezo-bev is contraindicated). Lenvatinib is non-inferior to sorafenib but has not been tested against atezo-bev[84]. Likewise, durvalumab monotherapy is non-inferior to sorafenib[75]. However, it is not yet clear what the best second line options are following lenvatinib or durvalumab monotherapy, hence sorafenib is preferred in current treatment algorithms[55].
For each of these medications, there are attendant considerations around adverse drug reactions, monitoring and drug interactions. For example, the TKIs and the multi-kinase inhibitors can lead to hand-foot syndrome, checkpoint inhibitors such as atezolizumab can cause acute hepatocellular and cholestatic liver injury, and almost all of the systemic therapies can cause common adverse events such as diarrhoea, rash and fatigue[73–75,81,82]. Consequently, pharmacists and pharmacy technicians are involved when initiating therapy, and during treatment. The systemic therapy landscape has changed rapidly over the past five years and it is likely to continue to evolve as more therapies are demonstrated to effectively treat HCC. This will make the role of pharmacists increasingly important, as more drug options become available, and we are able to individualise care to account for patient characteristics and concomitant treatments for pre-existing illness.
Best supportive care
Patients with advanced liver disease, who are outside of transplant criteria and are unsuitable for systemic therapy, should be offered best supportive care. It is recognised that often their prognosis is limited more by their advanced liver disease than by their cancer, and their care should include optimisation of such[15,33,55]. This includes management of hepatic encephalopathy and ascites, alongside more traditional palliative measures, such as control of pain, nausea and agitation. The use of questionnaires as quality-of-life screening tools can highlight primary symptoms to address from a patient perspective[85]. Increasingly, the importance of palliative care in the management of chronic liver disease is being recognised, and specific guidance is being developed to assist clinicians managing these challenging patients[86]. Many of the medicines traditionally used to control distressing symptoms at the end of life are contraindicated in those with advanced liver disease or can cause adverse effects; for example, opiates can provoke hepatic encephalopathy[87]. Doses may need close adjustment and monitoring, and alternative medications may need to be identified — for example, shorter rather than longer acting benzodiazepines for agitation may be preferred to prevent accumulation[88].
Summary
Pharmacists are closely involved at each stage of the patient’s journey with HCC. They are involved in optimising the treatment of underlying liver disease, including screening for drug interactions and adverse events in patients with chronic liver disease. The role of the pharmacy team is instrumental in monitoring immunosuppressant dosing in those undergoing transplant and in the preparation of chemo-embolic agents and immunotherapy.
Best practice points
- HCC usually arises in patients with chronic liver disease — this can be a result of viral hepatitis, alcohol-related liver disease or non-alcoholic fatty liver disease;
- Selected patients with chronic liver disease should undergo six-monthly ultrasound surveillance for HCC;
- The management of HCC depends on background liver function, as well as the stage of cancer;
- Resection and liver transplant are curative; medicines such as atezolizumab-bevacizumab and sorafenib are aimed at prolonging life expectancy but do not offer a cure.
- 1Rumgay H, Arnold M, Ferlay J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. Journal of Hepatology. 2022;77:1598–606. doi:10.1016/j.jhep.2022.08.021
- 2Fitzmaurice C, Allen C, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015. JAMA Oncol. 2017;3:524. doi:10.1001/jamaoncol.2016.5688
- 3Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2. doi:10.1038/nrdp.2016.18
- 4Liver cancer incidence statistics. Cancer Research UK. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/liver-cancer/incidence (accessed Apr 2023).
- 5Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021;71:7–33. doi:10.3322/caac.21654
- 6Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7. doi:10.1038/s41572-020-00240-3
- 7Akinyemiju T, Abera S, et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level. JAMA Oncol. 2017;3:1683. doi:10.1001/jamaoncol.2017.3055
- 8Kanwal F, Kramer JR, Mapakshi S, et al. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology. 2018;155:1828-1837.e2. doi:10.1053/j.gastro.2018.08.024
- 9Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2017;67:123–33. doi:10.1002/hep.29466
- 10Lee Y-CA, Cohet C, Yang Y-C, et al. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. International Journal of Epidemiology. 2009;38:1497–511. doi:10.1093/ije/dyp280
- 11Brown KF, Rumgay H, Dunlop C, et al. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br J Cancer. 2018;118:1130–41. doi:10.1038/s41416-018-0029-6
- 12Singal AG, Zhang E, Narasimman M, et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: A meta-analysis. Journal of Hepatology. 2022;77:128–39. doi:10.1016/j.jhep.2022.01.023
- 13Cucchetti A, Cescon M, Erroi V, et al. Cost-effectiveness of liver cancer screening. Best Practice & Research Clinical Gastroenterology. 2013;27:961–72. doi:10.1016/j.bpg.2013.08.021
- 14Singal AG, Yopp A, S. Skinner C, et al. Utilization of Hepatocellular Carcinoma Surveillance Among American Patients: A Systematic Review. J GEN INTERN MED. 2012;27:861–7. doi:10.1007/s11606-011-1952-x
- 15Galle PR, Forner A, Llovet JM, et al. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. Journal of Hepatology. 2018;69:182–236. doi:10.1016/j.jhep.2018.03.019
- 16Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2017;67:358–80. doi:10.1002/hep.29086
- 17Omata M, Cheng A-L, Kokudo N, et al. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–70. doi:10.1007/s12072-017-9799-9
- 18Singal AG, Yopp AC, Gupta S, et al. Failure Rates in the Hepatocellular Carcinoma Surveillance Process. Cancer Prevention Research. 2012;5:1124–30. doi:10.1158/1940-6207.capr-12-0046
- 19SINGAL A, VOLK ML, WALJEE A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Alimentary Pharmacology & Therapeutics. 2009;30:37–47. doi:10.1111/j.1365-2036.2009.04014.x
- 20Atiq O, Tiro J, Yopp AC, et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology. 2016;65:1196–205. doi:10.1002/hep.28895
- 21Nakhleh RE. The pathological differential diagnosis of portal hypertension. Clinical Liver Disease. 2017;10:57–62. doi:10.1002/cld.655
- 22Yang ZF, Poon RTP. Vascular Changes in Hepatocellular Carcinoma. Anat Rec. 2008;291:721–34. doi:10.1002/ar.20668
- 23Morse MA, Sun W, Kim R, et al. The Role of Angiogenesis in Hepatocellular Carcinoma. Clinical Cancer Research. 2019;25:912–20. doi:10.1158/1078-0432.ccr-18-1254
- 24Fung A, Shanbhogue KP, Taffel MT, et al. Hepatocarcinogenesis. Magnetic Resonance Imaging Clinics of North America. 2021;29:359–74. doi:10.1016/j.mric.2021.05.007
- 25Hanna RF, Miloushev VZ, Tang A, et al. Comparative 13-year meta-analysis of the sensitivity and positive predictive value of ultrasound, CT, and MRI for detecting hepatocellular carcinoma. Abdom Radiol. 2015;41:71–90. doi:10.1007/s00261-015-0592-8
- 26Aubé C, Oberti F, Lonjon J, et al. EASL and AASLD recommendations for the diagnosis of HCC to the test of daily practice. Liver Int. 2017;37:1515–25. doi:10.1111/liv.13429
- 27Tang M, Li Y, Lin Z, et al. Hepatic nodules with arterial phase hyperenhancement and washout on enhanced computed tomography/magnetic resonance imaging: how to avoid pitfalls. Abdom Radiol. 2020;45:3730–42. doi:10.1007/s00261-020-02560-0
- 28Kim JH, Joo I, Lee JM. Atypical Appearance of Hepatocellular Carcinoma and Its Mimickers: How to Solve Challenging Cases Using Gadoxetic Acid-Enhanced Liver Magnetic Resonance Imaging. Korean J Radiol. 2019;20:1019. doi:10.3348/kjr.2018.0636
- 29Di Martino M, De Filippis G, De Santis A, et al. Hepatocellular carcinoma in cirrhotic patients: prospective comparison of US, CT and MR imaging. Eur Radiol. 2012;23:887–96. doi:10.1007/s00330-012-2691-z
- 30Ricke J, Steffen IG, Bargellini I, et al. Gadoxetic acid-based hepatobiliary MRI in hepatocellular carcinoma. JHEP Reports. 2020;2:100173. doi:10.1016/j.jhepr.2020.100173
- 31Chernyak V, Fowler KJ, Kamaya A, et al. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology. 2018;289:816–30. doi:10.1148/radiol.2018181494
- 32EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. Journal of Hepatology. 2016;65:146–81. doi:10.1016/j.jhep.2016.03.005
- 33Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–50. doi:10.1002/hep.29913
- 34Silva MA, Hegab B, Hyde C, et al. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut. 2008;57:1592–6. doi:10.1136/gut.2008.149062
- 35Rockey DC, Caldwell SH, Goodman ZD, et al. Liver biopsy. Hepatology. 2008;49:1017–44. doi:10.1002/hep.22742
- 36Tayob N, Christie I, Richardson P, et al. Validation of the Hepatocellular Carcinoma Early Detection Screening (HES) Algorithm in a Cohort of Veterans With Cirrhosis. Clinical Gastroenterology and Hepatology. 2019;17:1886-1893.e5. doi:10.1016/j.cgh.2018.12.005
- 37Pepe MS, Etzioni R, Feng Z, et al. Phases of Biomarker Development for Early Detection of Cancer. JNCI Journal of the National Cancer Institute. 2001;93:1054–61. doi:10.1093/jnci/93.14.1054
- 38Farinati F, Marino D, De Giorgio M, et al. Diagnostic and Prognostic Role of alpha-Fetoprotein in Hepatocellular Carcinoma: Both or Neither? Am J Gastroenterology. 2006;101:524–32. doi:10.1111/j.1572-0241.2006.00443.x
- 39Tzartzeva K, Obi J, Rich NE, et al. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology. 2018;154:1706-1718.e1. doi:10.1053/j.gastro.2018.01.064
- 40Ikai I, Arii S, Kojiro M, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer. 2004;101:796–802. doi:10.1002/cncr.20426
- 41Zheng S-S, Xu X, Wu J, et al. Liver Transplantation for Hepatocellular Carcinoma: Hangzhou Experiences. Transplantation. 2008;85:1726–32. doi:10.1097/tp.0b013e31816b67e4
- 42Duvoux C, Roudot–Thoraval F, Decaens T, et al. Liver Transplantation for Hepatocellular Carcinoma: A Model Including α-Fetoprotein Improves the Performance of Milan Criteria. Gastroenterology. 2012;143:986-994.e3. doi:10.1053/j.gastro.2012.05.052
- 43Hong SK, Lee K-W, Kim H-S, et al. Living donor liver transplantation for hepatocellular carcinoma in Seoul National University. Hepatobiliary Surg. Nutr. 2016;5:453–60. doi:10.21037/hbsn.2016.08.07
- 44Marrero JA, Feng Z, Wang Y, et al. α-Fetoprotein, Des-γ Carboxyprothrombin, and Lectin-Bound α-Fetoprotein in Early Hepatocellular Carcinoma. Gastroenterology. 2009;137:110–8. doi:10.1053/j.gastro.2009.04.005
- 45Llovet J, Brú C, Bruix J. Prognosis of Hepatocellular Carcinoma: The BCLC Staging Classification. Semin Liver Dis. 1999;19:329–38. doi:10.1055/s-2007-1007122
- 46Roayaie S, Obeidat K, Sposito C, et al. Resection of hepatocellular cancer ≤2 cm: Results from two Western centers. Hepatology. 2013;57:1426–35. doi:10.1002/hep.25832
- 47Morise Z, Ciria R, Cherqui D, et al. Can we expand the indications for laparoscopic liver resection? A systematic review and meta-analysis of laparoscopic liver resection for patients with hepatocellular carcinoma and chronic liver disease. J Hepatobiliary Pancreat Sci. 2015;22:342–52. doi:10.1002/jhbp.215
- 48Lang H, Sotiropoulos GC, Dömland M, et al. Liver resection for hepatocellular carcinoma in non-cirrhotic liver without underlying viral hepatitis. British Journal of Surgery. 2004;92:198–202. doi:10.1002/bjs.4763
- 49Viganò L, Conci S, Cescon M, et al. Liver resection for hepatocellular carcinoma in patients with metabolic syndrome: A multicenter matched analysis with HCV-related HCC. Journal of Hepatology. 2015;63:93–101. doi:10.1016/j.jhep.2015.01.024
- 50Citterio D, Facciorusso A, Sposito C, et al. Hierarchic Interaction of Factors Associated With Liver Decompensation After Resection for Hepatocellular Carcinoma. JAMA Surg. 2016;151:846. doi:10.1001/jamasurg.2016.1121
- 51Mazzaferro V, Regalia E, Doci R, et al. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N Engl J Med. 1996;334:693–700. doi:10.1056/nejm199603143341104
- 52Llovet JM, Bruix J, Fuster J, et al. Liver transplantation for small hepatocellular carcinoma: The tumor-node-metastasis classification does not have prognostic power. Hepatology. 1998;27:1572–7. doi:10.1002/hep.510270616
- 53Bismuth H, Majno P, Adam R. Liver Transplantation for Hepatocellular Carcinoma. Semin Liver Dis. 1999;19:311–22. doi:10.1055/s-2007-1007120
- 54Jonas S. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080–6. doi:10.1053/jhep.2001.23561
- 55Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. Journal of Hepatology. 2022;76:681–93. doi:10.1016/j.jhep.2021.11.018
- 56Cucchetti A, Piscaglia F, Cescon M, et al. An explorative data-analysis to support the choice between hepatic resection and radiofrequency ablation in the treatment of hepatocellular carcinoma. Digestive and Liver Disease. 2014;46:257–63. doi:10.1016/j.dld.2013.10.015
- 57Cucchetti A, Piscaglia F, Cescon M, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. Journal of Hepatology. 2013;59:300–7. doi:10.1016/j.jhep.2013.04.009
- 58Lee SK, Chung DJ, Cho SH. A Real-World Comparative Study of Microwave and Radiofrequency Ablation in Treatment-Naïve and Recurrent Hepatocellular Carcinoma. JCM. 2022;11:302. doi:10.3390/jcm11020302
- 59Zhang Z, Yu J, Liu S, et al. Multiparametric liver MRI for predicting early recurrence of hepatocellular carcinoma after microwave ablation. Cancer Imaging. 2022;22. doi:10.1186/s40644-022-00471-5
- 60Lee DH, Lee JM, Lee JY, et al. Radiofrequency Ablation of Hepatocellular Carcinoma as First-Line Treatment: Long-term Results and Prognostic Factors in 162 Patients with Cirrhosis. Radiology. 2014;270:900–9. doi:10.1148/radiol.13130940
- 61Wai-To Lam V, Kwok-Chai Ng K, Siu-Ho Chok K, et al. Risk Factors and Prognostic Factors of Local Recurrence after Radiofrequency Ablation of Hepatocellular Carcinoma. Journal of the American College of Surgeons. 2008;207:20–9. doi:10.1016/j.jamcollsurg.2008.01.020
- 62Liao M, Zhong X, Zhang J, et al. Radiofrequency ablation using a 10-mm target margin for small hepatocellular carcinoma in patients with liver cirrhosis: A prospective randomized trial. J Surg Oncol. 2017;115:971–9. doi:10.1002/jso.24607
- 63Sasaki A, Kai S, Iwashita Y, et al. Microsatellite distribution and indication for locoregional therapy in small hepatocellular carcinoma. Cancer. 2005;103:299–306. doi:10.1002/cncr.20798
- 64Llovet JM, De Baere T, Kulik L, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:293–313. doi:10.1038/s41575-020-00395-0
- 65Galle PR, Tovoli F, Foerster F, et al. The treatment of intermediate stage tumours beyond TACE: From surgery to systemic therapy. Journal of Hepatology. 2017;67:173–83. doi:10.1016/j.jhep.2017.03.007
- 66Salem R, Lewandowski RJ. Chemoembolization and Radioembolization for Hepatocellular Carcinoma. Clinical Gastroenterology and Hepatology. 2013;11:604–11. doi:10.1016/j.cgh.2012.12.039
- 67Kim N, Cheng J, Jung I, et al. Stereotactic body radiation therapy vs. radiofrequency ablation in Asian patients with hepatocellular carcinoma. Journal of Hepatology. 2020;73:121–9. doi:10.1016/j.jhep.2020.03.005
- 68Rajyaguru DJ, Borgert AJ, Smith AL, et al. Radiofrequency Ablation Versus Stereotactic Body Radiotherapy for Localized Hepatocellular Carcinoma in Nonsurgically Managed Patients: Analysis of the National Cancer Database. JCO. 2018;36:600–8. doi:10.1200/jco.2017.75.3228
- 69Choi HS, Kang KM, Jeong BK, et al. Effectiveness of stereotactic body radiotherapy for portal vein tumor thrombosis in patients with hepatocellular carcinoma and underlying chronic liver disease. Asia‐Pac J Clin Oncol. 2020;17:209–15. doi:10.1111/ajco.13361
- 70Eriguchi T, Takeda A, Sanuki N, et al. Acceptable Toxicity After Stereotactic Body Radiation Therapy for Liver Tumors Adjacent to the Central Biliary System. International Journal of Radiation Oncology*Biology*Physics. 2013;85:1006–11. doi:10.1016/j.ijrobp.2012.09.012
- 71Yao FY, Mehta N, Flemming J, et al. Downstaging of hepatocellular cancer before liver transplant: Long‐term outcome compared to tumors within Milan criteria. Hepatology. 2015;61:1968–77. doi:10.1002/hep.27752
- 72Mazzaferro V, Citterio D, Bhoori S, et al. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. The Lancet Oncology. 2020;21:947–56. doi:10.1016/s1470-2045(20)30224-2
- 73Cheng A-L, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. Journal of Hepatology. 2022;76:862–73. doi:10.1016/j.jhep.2021.11.030
- 74Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894–905. doi:10.1056/nejmoa1915745
- 75Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. JCO. 2022;40:379–379. doi:10.1200/jco.2022.40.4_suppl.379
- 76Hollebecque A, Cattan S, Romano O, et al. Safety and efficacy of sorafenib in hepatocellular carcinoma: the impact of the Child-Pugh score. Alimentary Pharmacology & Therapeutics. 2011;34:1193–201. doi:10.1111/j.1365-2036.2011.04860.x
- 77Abou-Alfa G, Amadori D, Santoro A, et al. Safety and Efficacy of Sorafenib in Patients with Hepatocellular Carcinoma (HCC) and Child-Pugh A versus B Cirrhosis. Gastrointest Cancer Res 2011;4:40–4.https://www.ncbi.nlm.nih.gov/pubmed/21673874
- 78Xiao B, Wang W, Zhang D. Risk of bleeding associated with antiangiogenic monoclonal antibodies bevacizumab and ramucirumab: a meta-analysis of 85 randomized controlled trials. OTT. 2018;Volume 11:5059–74. doi:10.2147/ott.s166151
- 79Ollivier-Hourmand I, Allaire M, Cervoni JP, et al. Management of portal hypertension in patients treated with atezolizumab and bevacizumab for hepatocellular carcinoma. Journal of Hepatology. 2022;77:566–7. doi:10.1016/j.jhep.2022.02.004
- 80de Franchis R, Bosch J, Garcia-Tsao G, et al. Baveno VII – Renewing consensus in portal hypertension. Journal of Hepatology. 2022;76:959–74. doi:10.1016/j.jhep.2021.12.022
- 81Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. 2017;389:56–66. doi:10.1016/s0140-6736(16)32453-9
- 82Abou-Alfa GK, Meyer T, Cheng A-L, et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379:54–63. doi:10.1056/nejmoa1717002
- 83Zhu AX, Kang Y-K, Yen C-J, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Oncology. 2019;20:282–96. doi:10.1016/s1470-2045(18)30937-9
- 84Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. The Lancet. 2018;391:1163–73. doi:10.1016/s0140-6736(18)30207-1
- 85Berry P, Kotha S. Improving end of life care in liver disease. Journal of Hepatology. 2022;76:1225–6. doi:10.1016/j.jhep.2021.10.001
- 86Rogal SS, Hansen L, Patel A, et al. AASLD Practice Guidance: Palliative care and symptom‐based management in decompensated cirrhosis. Hepatology. 2022;76:819–53. doi:10.1002/hep.32378
- 87Chandok N, Watt K. Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc 2010;85:451–8. doi:10.4065/mcp.2009.0534
- 88Peppers M. Benzodiazepines for alcohol withdrawal in the elderly and in patients with liver disease. Pharmacotherapy 1996;16:49–57.https://www.ncbi.nlm.nih.gov/pubmed/8700792