This content was published in 2011. We do not recommend that you make any clinical decisions based on this information without first ensuring you have checked the latest guidance
Key points
- Immunosuppressants are used in transplantation to prevent early rejection (induction), to prevent long-term rejection (maintenance) and to treat rejection.
- Immunosuppression is a balance between prevention of rejection and the increased risk of side effects.
- Complications of transplantation include infection, new onset diabetes after transplant and malignancy.
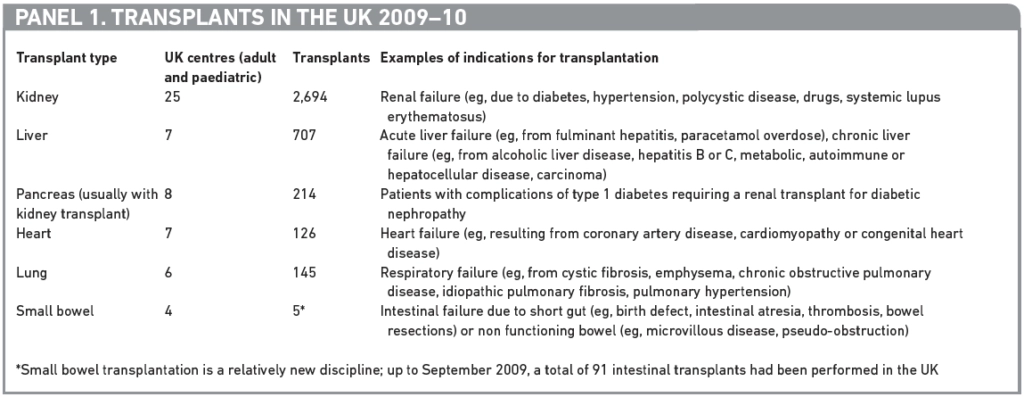
There were over 3,700 solid organ transplants carried out in 59 centres throughout the UK in 2009 to 2010. The solid organ transplants performed include kidney, liver, heart, lung, pancreas and small bowel. In some cases two or more organs are transplanted. Panel 1 outlines the types of transplant, their approximate numbers and examples of conditions resulting in organ failure.
Despite the rising number of transplants, there are still over 7,500 patients waiting for an organ and patients continue to die on the waiting list. One aim of NHS Blood and Transplant (NHSBT; a special health authority responsible for optimising the supply of blood, organs and tissues, and raising the quality, effectiveness and efficiency of blood and transplant services) is that the number of transplants in the UK exceeds 4,800 by 2014. This can only be achieved by increasing the number of organs donated and the British Transplant Society is working to achieve this through increasing the number of live donations, increased use of non-heart beating donors and via NHSBT schemes such as creating an organ donor task force.
Criteria for accepting patients onto a transplant waiting list generally include:
- The need for the transplant (eg, if life expectancy without a transplant is significantly reduced, other surgical or medical options for treatment are unsuitable, and quality of life is significantly reduced);
- That the patient is of suitable fitness to endure the surgical procedure;
- That a transplant will offer increased survival and quality of life.
Allocation of organs depends on a number of factors, such as tissue typing compatibility between the donor and recipient (for renal transplants), blood group compatibility (for lung, heart and liver transplants), size of the organ, recipient age, days on the waiting list and recipient location. Success rates achieved following solid organ transplants are detailed in Panel 2.

Therapies used in transplantation
Therapies in transplantation are based on immunosuppression but include prophylaxis.
Immunosuppression
One of the first major breakthroughs in transplantation occurred in the late 1940s when Sir Peter Medawar identified the immune system and stated that transplantation would not be successful unless this system was suppressed.
In the early 1960s prednisolone was found to be immunosuppressive, followed by 6-mercaptopurine, azathioprine and antithymocyte immunoglobulin (ATG). These agents allowed increased success in transplantation.
It was then discovered that a combination of agents lead to improved immunosuppression with a reduction in side effects, and patients are still treated today — in the early period post transplant at least —with a combination of immunosuppressive agents rather than a single agent.
The next major breakthrough came with the discovery of ciclosporin in the late 1960s. Ciclosporin, along with improvements in surgical and organ retrieval techniques, significantly improved transplantation success rates and allowed transplantation to move from an experimental treatment to an approved therapeutic option. Since the discovery of ciclosporin, the number of immunosuppressive agents available has increased dramatically and drugs such as tacrolimus, sirolimus, basiliximab, mycophenolate and alemtuzumab are now used routinely within transplantation to prevent and treat organ rejection.
The main site of action for the immunosuppressive agents is T-lymphocyte cells. Some agents also have activity against the B lymphocyte. Figure 1 shows the mechanism of transplant rejection mediated by the T-lymphocyte cell along with the site of action of the immunosuppressive agents. The recipient’s immune system identifies the transplant as foreign through interaction with antigen-presenting cells. Signals 1 and 2 are triggered, which in turn activates the T-lymphocyte cell, resulting in an increased production of T-cells that attack the transplanted tissue.
Unless a transplant has been carried out between genetically identical twins, immunosuppression is necessary. The use of immunosuppressive agents in transplantation can be divided into use in three areas:
- Induction (to reduce the likelihood of acute rejection in the early period following transplantation);
- Maintenance (to prevent rejection in the long term);
- Treating rejection (this is discussed in ‘How we care for kidney transplant patients’[needs link adding when both live]).
Regimens used for both induction and maintenance will vary between types of transplant and between patient groups within the same transplant type. For example, a patient receiving a liver transplant because of hepatitis B or C will require less immunosuppression than a recipient with autoimmune liver disease. Patients requiring a liver transplant have a lower risk of rejection (since liver is continually exposed to antigenic loads, it is able to induce immunity or tolerance to antigens, unlike other organs) and so will require less immunosuppression than a patient requiring a heart transplant.

Induction therapy
Induction therapy is given at the time of transplantation. Medicines licensed for use as an induction therapy are corticosteroids, basiliximab and ATG. Alemtuzumab is also used for induction although it is not licensed for this indication.
Basiliximab
Basiliximab (Simulect) is a lymphocyte depleting anti-CD25 monoclonal antibody licensed for use as an induction agent in adult and paediatric renal transplants, and is recommended for such by the National Institute for Health and Clinical Excellence1.
It is also often used (unlicensed) as an induction agent in other types of transplant, for example in liver patients coming to transplant with renal impairment basiliximab allows a delay in initiation of the potentially nephrotoxic calcineurin inhibitor (i.e., tacrolimus, ciclosporin) allowing the kidney time to recover in the immediate post transplant period. Its mechanism of action is to block the interleukin 2 receptor (also known as the CD25 antigen), preventing activation of T-lymphocyte cells. Basiliximab is administered intravenously as two doses, one before or just after transplant and one at day 4 post transplant.
ATG
ATG (Thymoglobuline) is licensed for both induction therapy and for treatment of acute steroid-resistant rejection. ATG binds to the main receptor of the T-cells and activates them before destroying them. This causes the release of cytokines, which can produce an inflammatory response with fever, chills, shaking and hypotension. Lymphopenia is also a common side effect of ATG. In the UK, ATG is more commonly used to treat steroidresistant rejection than as an induction agent.
Alemtuzumab
Alemtuzumab (MabCampath) is a potent lymphocyte-depleting anti-CD52 monoclonal antibody. Its use within transplantation is unlicensed, but it offers potent induction and has been used within transplantation for a number of years. It is increasingly being used for transplants where higher levels of immunosuppression are required, such as in small bowel transplants, and as an induction agent for steroid-avoiding regimens (e.g., in patients with diabetes).
A national renal transplant study, called the 3C study, has recently started recruiting to compare basiliximab and alemtuzumab as induction agents in renal transplantation.
The first dose of alemtuzumab is given at the time of transplant. A second dose may or may not be given after transplant. Intravenous administration is often associated with a first dose cytokine syndrome characterised by fever and hypotension so the drug is often given by subcutaneous injection. Subcutaneous administration results in similar blood levels and efficacy to intravenous administration with fewer side effects2.
Maintenance
Transplant recipients will require maintenance immunosuppression for the life of the graft, in order to prevent rejection and maintain graft survival. The medicines used in long-term maintenance therapy are ciclosporin, tacrolimus, corticosteroids, azathioprine, mycophenolate and sirolimus.
Ciclosporin and tacrolimus
Ciclosporin and tacrolimus are the gold standard of modern immunosuppression. They prevent early T-cell activation by inhibiting calcineurin, an enzyme involved in the transcription of genes encoding interleukin-2 and other cytokines.
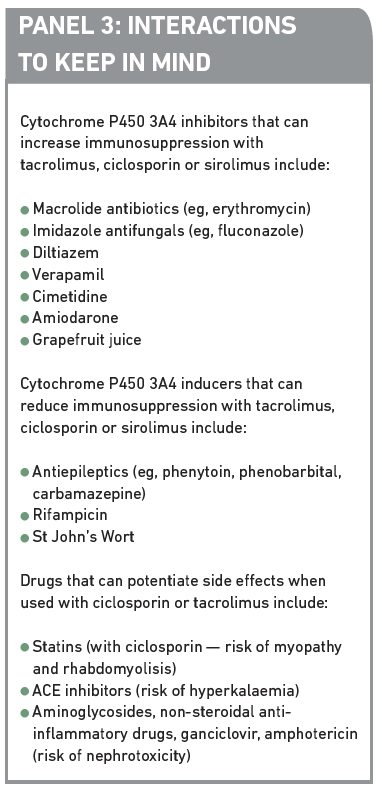
They are both metabolised in the liver and bowel under the influence of cytochrome P450 3A4 enzymes and the p-glycoprotein efflux pump so pharmacokinetic drug interactions are common. Other potential interactions also need to be considered, for example, with other medicines that may exacerbate the side effects of ciclosporin or tacrolimus. Examples of potential interactions are given in Panel 3. Monitoring for, and advising on potential interactions with immunosuppressant medication is an important role for the pharmacist.
The pharmacokinetics of ciclosporin and tacrolimus show high inter-patient variations. They are both narrow therapeutic index drugs and hence therapeutic drug monitoring is routinely used to optimise dosing for individual patients in order to prevent graft rejection and minimise side effects. In the early period post transplant higher blood concentrations are aimed for with time. As the risk of rejection decreases, lower therapeutic blood concentrations are aimed for.
The main adverse effects of ciclosporin and tacrolimus are nephrotoxicity, neurotoxicity and glucose intolerance. Tacrolimus is more likely to cause neurotoxicity (e.g., tremor and headache) and glucose intolerance than ciclosporin. Ciclosporin is more likely to cause hirsutism and gum hypertrophy.
Nephrotoxicity is closely monitored for. If it occurs the dose is reduced or the drug withdrawn and replaced with a non-nephrotoxic drug (eg, mycophenolate). In some cases calcineurin-induced renal failure can lead to a further renal transplant.
A number of generic ciclosporin and tacrolimus preparations are now available in the UK. These preparations are not interchangeable without specialist supervision and monitoring, and it is critical that patients are not inadvertently switched from one preparation to another because this can lead to blood concentrations falling outside the therapeutic range, resulting in the risk of rejection and graft loss or adverse side effects. Alerts to this effect have been issued by the Medicines Healthcare Products Regulatory Authority.
Corticosteroids
Prednisolone is the main corticosteroid used after transplantation. It is anti-inflammatory and affects most of the cells associated with initiation of a rejection episode. In maintenance doses corticosteroids block the release and inhibit the action of cytokine interleukins and interfere with T-cell activation.
Although corticosteroids are good at preventing transplant rejection they are associated with many side effects, such as cataracts, bone disease, Cushing’s syndrome, diabetes and hyperlipidaemia. Because of these side effects many transplant centres aim to use the lowest possible doses wherever appropriate. Corticosteroids are withdrawn as early as possible, leaving patients on single or double therapy (eg, ciclosporin/ tacrolimus and/or azathioprine/mycophenolate and/or sirolimus). Patients on long-term corticosteroid therapy will often need other treatments to minimise the risk of complications (eg, bisphosphonates for osteoporosis).
Azathioprine
Azathioprine was first introduced into clinical practice in the late 1950s and has been key in most immunosuppression protocols since then. Following administration, it is extensively and rapidly metabolised to 6-mercaptopurine (6-MP), which is further metabolised to thioguanine nucleotides. It acts as an antimetabolite, reducing DNA and RNA synthesis and blocking proliferation of lymphocytes.
The main route of 6-MP elimination is via the enzyme xanthine oxidase. This is blocked by xanthine oxidase inhibitors such as allopurinol. Hence allopurinol should only be used with caution in patients on azathioprine (a 75 per cent dose reduction of azathioprine is recommended). In practice, patients needing allopurinol will often be prescribed mycophenolate instead of azathioprine.
The main side effect of azathioprine is dose-dependent depression of bone marrow. Other side effects include nausea, alopecia and elevation of liver function tests.
Mycophenolate mofetil
Mycophenolate mofetil is a prodrug. Following administration it is metabolised via esterases to mycophenolic acid (MPA). This acts by inhibiting the enzyme inosine monophosphate dehydrogenase, leading to a blocking of guanosine synthesis and B- and T-cell proliferation. MPA is further metabolised via glucoronidation to mycophenolic acid glucoronide (MPAG) and eliminated via renal tubular secretion in the kidneys. Levels of drugs eliminated by renal tubular secretion (eg, ganciclovir) may be increased when given concurrently with mycophenolate.
Blood levels of MPA can be affected by ciclosporin, tacrolimus and sirolimus. Ciclosporin reduces MPA levels through enterohepatic recirculation. Tacrolimus and sirolimus potentially increase MPA levels. The main adverse effects of mycophenolate are gastrointestinal and haematological. Mycophenolate causes more vomiting, diarrhoea and anaemia than azathioprine but less leucopenia. An enteric coated preparation of mycophenolate (Myfortic), which potentially has an improved gastrointestinal side effect profile, is available.
Sirolimus
Sirolimus (Rapamune) was formerly known as rapamycin because it was discovered on Easter Island (Rapa Nui). It has a similar chemical structure to tacrolimus and binds to the same protein on the surface of the T-cell (FK12 binding protein) but the complex formed does not affect the calcineurin enzyme. Instead, it exerts its immunosuppressive effect later in the cell cycle by binding to the mammalian target of rapamycin (mTOR) and inhibiting IL-2 mediated signal transduction pathways. Sirolimus is metabolised via the cytochrome P450 3A4 enzymes so has similar drug pharmacokinetic interactions to the calcineurin inhibitors.
Sirolimus has the advantage of a different side effect profile to the calcineurin inhibitors. It does not cause nephrotoxicity and so offers a therapeutic option for transplant recipients with renal impairment. It is, however, associated with delayed wound healing (hence is not used immediately post transplant, and will only be introduced once the transplant wound has healed), lymphocele formation, hyperlipidaemia, hypertriglyceridaemia, leukopenia, anaemia, joint pain, mouth ulceration and pneumonitis.
Sirolimus has the added advantage that it may slow tumour cell proliferation. It may delay the progression of liver fibrosis in patients with hepatitis C. Studies investigating its effects in these areas are ongoing. Sirolimus is useful in kidney transplant recipients with haemolytic uraemic syndrome because this condition is likely to recur in the transplanted kidney if a calcineurin inhibitor is used.
Complications
All immunosuppression is a balance between providing enough of it, to prevent graft rejection, and not too much of it, to avoid side effects and complications such as infection and malignancy.
Infection
Infections occur in a generally predictable pattern after solid organ transplantation, as detailed in Figure 2. Development of infection is delayed by prophylaxis and accelerated by intensified immunosuppression. Common infections and corresponding prophylactic regimens are detailed in Panel 4.


New onset diabetes after transplant (NODAT)
The prevalence of diabetes post transplantation is approximately six times that of age- and sex-matched controls. NODAT incurs high risks, notably increased morbidity and mortality.
After transplantation insulin sensitivity improves, although many patients (over 40 per cent) remain insulin-resistant. Changes to the immunosuppressive regimen can help to alleviate diabetes. These include withdrawing or reducing the dose and duration of corticosteroids, switching tacrolimus to ciclosporin or withdrawing the calcineurin inhibitor altogether.
Malignancy
Many forms of cancer have a much higher incidence in transplant recipients than in the general population, especially non-melanoma skin cancers, cancer of the lip, post-transplant lymphoproliferative disease (PTLD), cancer of the oral cavity, cervical cancer and anal cancer. Skin cancer is the most common cancer affecting solid organ transplant recipients affecting up to 70 per cent of patients within 20 years post transplant. Possible reasons include that immunosuppression impairs immunological surveillance and that some immunosuppressants are linked to oncogenic effects (eg, azathioprine sensitises cells to UVA-induced damage).
All transplant patients must be counselled on the importance of sun protection and provided with a high factor sun block if necessary. Female patients should be advised to have an annual cervical smear test.
New drug developments
Belatacept has recently been granted marketing authorisation from the European Commission for use to prevent rejection in adult renal transplant recipients. This is an exciting development because it has a new mode of action: blocking CD28-mediated T-cell activation by binding CD80 and CD86 thus inhibiting the co-stimulatory pathway. Belatacept helps to preserve renal function post renal transplantation3. Potential concerns, however, are the development of PTLD and progressive multifocal leukoencephalopathy.
Eculizumab is a humanised anti C5 antibody that acts as a terminal complement inhibitor. It is licensed in the UK for treatment of paroxysmal nocturnal haemoglobinuria. There are reports in small numbers of patients that it is effective in reducing the need for plasma exchange post renal transplant and the rate of acute rejection is low4. However a high proportion of eculizumab patients showed signs of chronic renal injury within seven months of a transplant. Eculizumab offers a potential beneficial option in transplant patients with haemolytic uraemic syndrome.
The citation information is currently unavailable. For more information please contact pharmpress-support@rpharms.com