
TEK IMAGE/SCIENCE PHOTO LIBRARY
After reading this article, you should be able to:
- Describe the name, type, mechanism of action and landmark clinical trials of licensed advanced therapy medicinal products (ATMPs) currently approved for use in the UK;
- Explore processes for predicting future ATMPs;
- Establish the role of pharmacy professionals, including the chief pharmacist, clinical pharmacy professional and quality assurance specialist in the preparation and administration of ATMPs, and the pharmacy infrastructure needed for ATMPs;
- Describe the patient and product journey for an ATMP, from production to administration, with a focus on chimeric antigen receptor T-cell therapies;
- Depict ATMP regulatory processes;
- Establish the process for assessing cost-effectiveness and reimbursement strategies for ATMPs in the UK.
Advanced therapy medicinal products (ATMPs) are a new class of medicinal products. They include gene therapies, somatic cellular products and tissue engineered products[1]. ATMPs are at the forefront of healthcare innovation and give hope for treating diseases that currently have limited or no therapeutic options.
There are three principal categories of ATMPs:
- Medicines for gene therapy — these incorporate genes that can bring about an effect that is therapeutic, diagnostic or prophylactic. These products insert into the body so-called ‘recombinant’ genes (i.e. stretches of DNA, assembled from different sources and produced in a laboratory setting) with the objective of treating conditions such as genetic disorders, cancer or other chronic diseases[1]. Gene therapies that are virus-based are known as in vivo gene therapies, with genetic modification to the cells and tissues occurring inside the body after administration. Gene therapies that are cell-based are known as ex vivo gene therapies. They use a sample of donor cells that undergo genetic modification in a good manufacturing practice (GMP) manufacturing unit.
- Products based on somatic cell therapy — these comprise either substantially manipulated cells or tissues, or cells and tissues in which the biological characteristics have been altered to produce actions distinct from their normal function. These medicines are intended to cure, prevent or diagnose a disease[1].
- Tissue-engineered products — these are based on modified cells or tissues with the objective of repairing, replacing or regenerating human tissue. Cells embedded in a biodegradable matrix or scaffold represent an example of this type of product[1].
Medicines referred to as ‘combined ATMPs’ may incorporate a medical device as an integral component of the medicine.
ATMPs present challenges and opportunities. For scientists and healthcare professionals employed in commissioned ATMP delivery sites, challenges include ATMP preparation, storage and prescription; for those in regulatory sciences and pharmacy professionals in heath economic settings, challenges include licensing, processes and assessing ATMP cost-effectiveness[2]. However, ATMPs also bring hope, opportunity and clinical optimism to diagnoses that previously had devastating prognoses, such as adult B-cell lymphoma and resistant acute lymphocytic leukaemia in children[3].
The aim of this article is to provide an overview for the pharmacy professional of the clinical, institutional, regulatory, safety and economic considerations of ATMPs.
ATMPs licensed in the UK
At the time of writing, there are ten licensed ATMPs approved by the National Institute for Health and Care Excellence (NICE) for use in England and Wales. They are used in several indications, including blood cancers, solid tumour cancers, and metabolic, eye, immune, musculoskeletal and neuromuscular disorders[4]. Seven of these ATMPs are approved for eight indications by the Scottish Medicines Consortium for use in Scotland. For more detailed information, see Table 1 (click here for a PDF version)[5–15].
Horizon scanning for ATMPs
Horizon scanning is a systematic process of identifying new medicines in development. It facilitates early patient access to new medicines by anticipating hurdles in implementation. Horizon-scanning activities are undertaken by NHS medicines information specialists and published annually by the Specialist Pharmacy Service[16]. This is an essential service for NHS organisations, enabling the planning and implementing of budgets for new medicines or licence extensions predicted within 18 months. The 2021 publication predicted 21 new ATMPs and 8 ATMP licence extensions over the next 18 months[17].
The Cell and Gene Therapy Catapult (CGTC) is an expert UK centre, established and designed to transform the UK’s capability for innovation and support economic growth in ATMPs[18]. The CGTC encourages collaborative working with industry to break down barriers in the pipeline of ATMPs. It also manages a comprehensive clinical trials database that contains details of all UK trials of ATMPs. There were 214 ongoing trials in 2021[19]. The dominant fields are haematological malignancies and solid tumours, accounting for around 35% of ATMP trials, followed by haematology and ophthalmology at 12% each (see Figure 1)[19–29].
The role of pharmacy professionals
Pharmacy professionals engage with ATMPs in several areas, from specialist critical care pharmacists that review patients with neurotoxicity after ATMP administration to pharmacists as qualified persons that ensure appropriate governance in ATMP production.
Governance, chief pharmacists and ATMPS
Chief pharmacists are responsible for the safe and secure handling and quality of medicinal products within their organisation[18]. The first guidance for pharmacy leaders on handling ATMPs was published in 2017 by the Specialist Pharmacy Service and advocated appropriate medicines governance for ATMPs within healthcare organisations[19].
The guidance stipulated a collaborative approach to ensure safe ATMP introduction. Chief pharmacists recognise that the handling and manipulation of cellular medicines is a specialist competency outside the pharmacy workforce’s skill set. However, it remains a pharmacy responsibility to ensure proper procedures for the manipulation of ATMPs in compliance with good clinical practice for investigational medicinal products (IMP) or the summary of prescribing characteristics for marketed medicines. Pharmacists should also facilitate multidisciplinary collaboration so that pharmacovigilance responsibilities are fully delivered. The ultimate responsibility for all safe and compliant procedures in relation to medicines (including ATMPs) sits with the chief pharmacist[19].
All requests for ATMPs must be scrutinised by the appropriate organisational multidisciplinary committee to ensure stakeholders are aware of their associated effects. For example, there may be a requirement for specialist services, such as an intensive care unit for management of serious adverse effects after ATMP administration. Importantly, for advanced therapy investigational medicinal products (ATIMPs) used in a clinical trial, there are additional local governance steps, in addition to approval via research and development.
Additional national governance processes for ATMPs holding marketing authorisations are managed centrally through one of the following:
- A NICE health technology evaluation;
- Highly specialised technologies evaluation processes;
- NHS England commissioning process (comparable processes exist in other UK countries).
Currently, delivery centres for marketed ATMPs must be commissioned by the NHS England national process [30]. However, there is no such process for sites to become eligible to deliver clinical trials.
The eligibility of potential patients may be assessed centrally by a multidisciplinary approval panel process, as is the case for chimeric antigen receptor T-cell (CAR-T) therapy.
Should a cellular autologous ATMP fail to meet criteria for final release as a licensed medicine or IMP, it will be subject to additional governance requirements. However, there are circumstances in which the treatment may still be administered. GMP for ATMPs allows, in exceptional circumstances, for the concessionary release of products that do not comply with release specifications[31,32]. A product can be released for use on a compassionate basis, at the request of the clinician, as ATMPs can give the patient a last chance of survival. There are liability issues in these circumstances: where the product does not full comply with marketing authorisation, liability rests with the prescriber and their institution rather than the manufacturer[33]. Local governance should ensure that clinical risks and benefits for the individual patient are considered before acceptance of an out-of-specification ATMP[33].
Local ATMP policies should ensure the ATMP is assessed as suitable by a quality assurance pharmacist with relevant expertise (similar to what is required for unlicensed medicine use)[32]. The NHS workforce includes qualified persons (as defined in Directive 2001/83/EC) and quality assurance specialists who assist with ATMP assessment in collaboration with stem cell laboratory colleagues[19,31].
Operational pharmacy role
When introducing an ATMP, the journey of the product and the patient should be carefully mapped. For some products, such as in vivo gene therapies, the pathways are similar to those for other medicines. However, some autologous cellular ATMPs; for example, ex vivo gene therapies such as CAR-T, are disruptive to ‘business as usual’ and require careful planning. CAR-T cell therapies are currently the most widely used ATMPs across the UK and will be discussed below to illustrate the complex patient and product journey.
Clinical pharmacy role
The role of the clinical pharmacist in ATMP provision has evolved and formalised since the advent of marketed CAR-T cell therapies, with focus on patient verification, linking with referral centres for medicines reconciliation and toxicity management[34]. The Pan-UK ATMP Pharmacy Working Group has a clinical pharmacy subgroup, which has produced a standardised toxicity management tool and consensus guidance for supportive medications, based on evidence and clinical experience[35]. The clinical pharmacy subgroup also aims to use horizon-scanning data to enable proactive collaboration and facilitate implementation of new ATMPs.
In addition, the Pan-UK ATMP Pharmacy Working Group has established a subgroup for clinical trials, which aims to ensure that pharmacy trials teams are equipped to ask appropriate questions at various stages, from first contact to establishing trial feasibility through site initiation, and into recruitment. Outputs are published on the NHS Specialist Pharmacy Service website[16,36].
In any setting, the clinical pharmacist has the responsibility to make patients aware of open-for-recruitment ATMP clinical trials for their specific condition. These can be found at www.clinicaltrial.gov.uk, www.catapult.org.uk/clinicail-trials-database and www.eudract.ema.europa.eu.
Patient and product journey for CAR-T
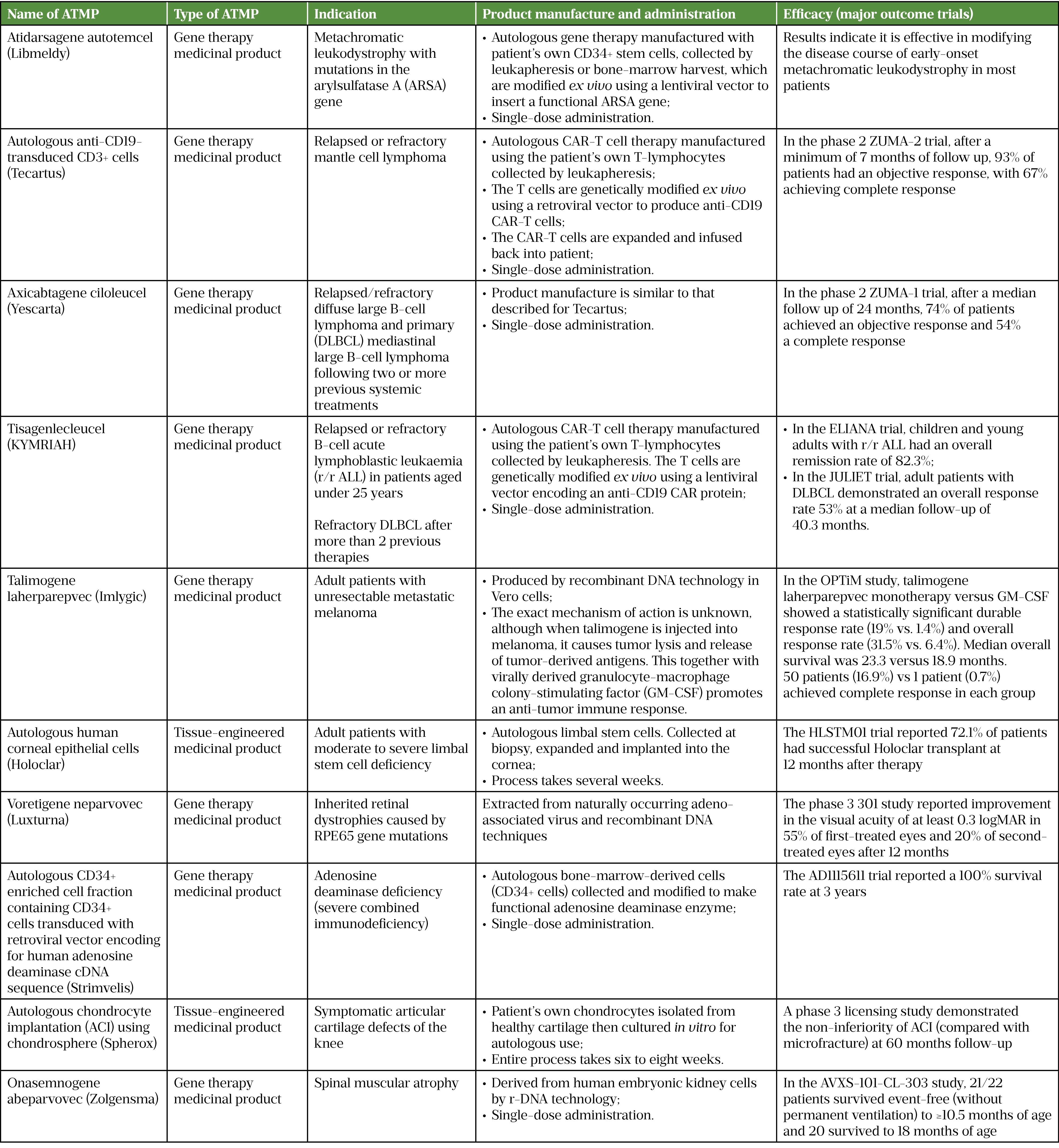
CAR-T therapy is the most commonly used ATMP in the UK. The CAR-T patient journey is outlined in Figure 2. It begins with a potentially eligible patient being referred to a CAR-T centre. If suitable, their case is submitted for discussion at a national panel, and funding approved at that point (if they are eligible for treatment)[37]. After consent is obtained, the patient attends the apheresis unit at the CAR-T centre, where their T-cells are collected by a process known as leukapheresis.
The patient may need bridging therapy to stabilise their disease while the CAR-T cell product is manufactured, as this can take 3–4 weeks[6]. Once the CAR-T cell product is manufactured and available for use, the patient is administered lymphodepletion (usually containing fludarabine and cyclophosphamide) to aid the efficacy and persistence of CAR-T cell therapy[7]. The patient is then admitted to hospital for the CAR-T cell infusion. Toxicity, such as cytokine release syndrome and neurotoxicity, can occur which would require careful management and (potentially) admission to an intensive care unit[38].
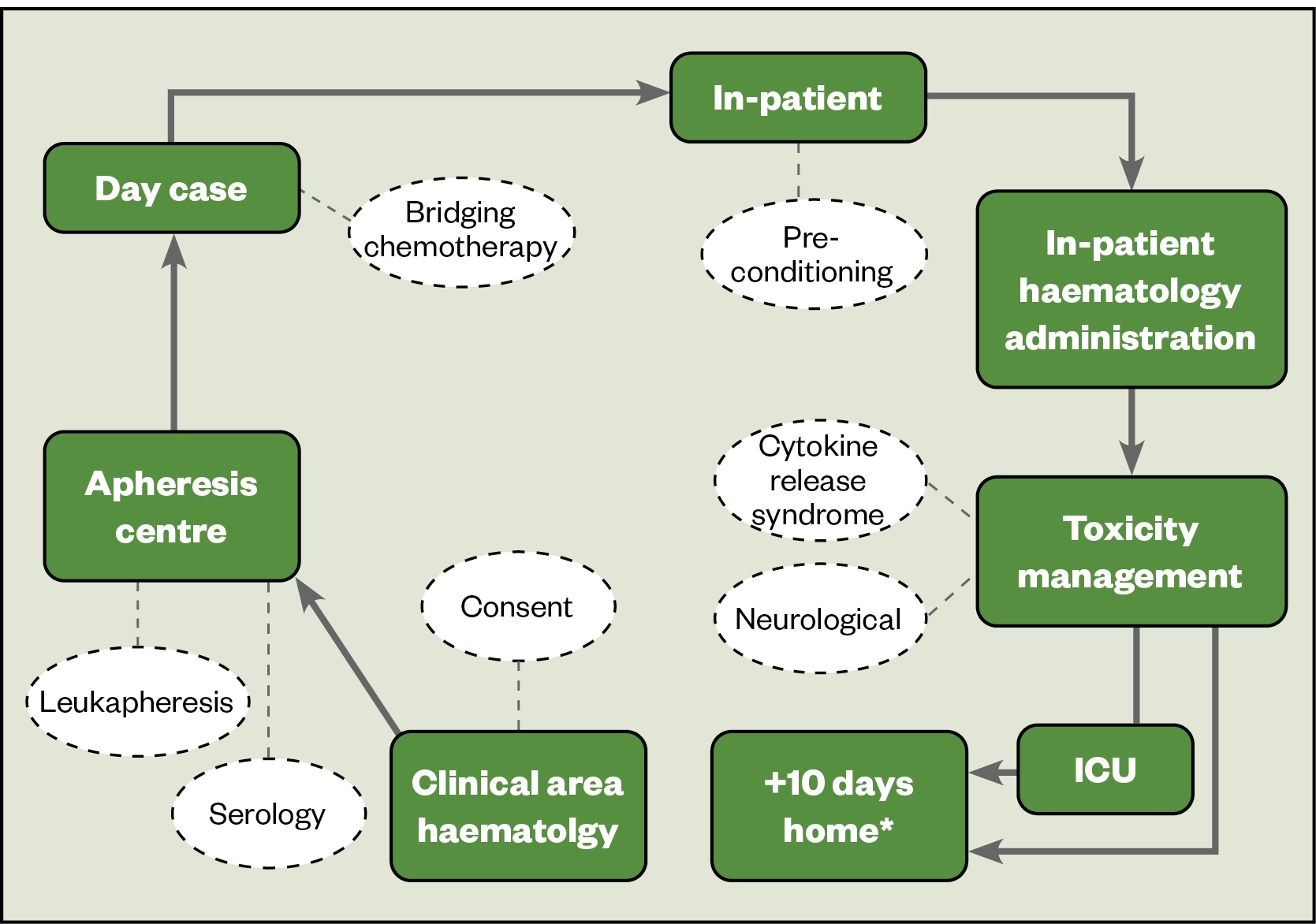
Figure 3 shows the product journey for CAR-T therapy. The starting product is harvested from the patient or a donor in the apheresis centre by a process called leukapheresis and processed in a stem cell laboratory. It is subsequently packaged and shipped to the manufacturer, where regulatory controls shift from the Human Tissue Authority in the UK to the Medicines and Healthcare products Regulatory Agency (MHRA) or the medicines regulator operating in the country of manufacture.
The starting material is then used in the manufacturing process, where it is genetically modified to confer antigen specificity to the T-cells by the addition of a chimeric antigen receptor[39]. After expansion, the product is classed a medicine. The medicinal product is cryopreserved and returned to the hospital via a stem cell laboratory, with pharmacy oversight. The quality is verified (right patient, right dose, product integral, no cold chain breach and stored under nitrogen until required)[19]. Prior to use, it is thawed and then immediately administered to the patient. The medicinal product requires specialist handling, and each centre has its own optimal process to ensure good clinical governance.
To ensure consistency and minimise variation in service provision, the Pan-UK ATMP Pharmacy Working Group and NHS Specialist Pharmacy Service have checklists for each stage requiring pharmacy input, which include key points to ensure standardised processes in each institution[40]. The NHS Specialist Pharmacy Service has published a guide for chief pharmacists implementing CAR-T therapy: ‘Pharmacy Institutional Readiness for Marketed CAR-T Therapies: Checklists for Pharmacy Services‘[19].
ATMP regulatory framework
In the UK and Europe, ATMPs are defined by the MHRA and European Medicines Agency (EMA) as medicines for use in humans, whose fundamental basis are genes, cells or tissues. In the USA, cell therapy and human gene therapy products, as well as combination devices involving cell and gene therapy, are regulated by the Food and Drug Administration (FDA)’s Center for Biologics Evaluation and Research.
Both cellular and gene therapy medicines are approved in Europe, UK and the United States.
ATMP cost-effectiveness in the context of NHS budgets
A main feature of ATMPs is their price. Tisagenlecleucel (KYMRIAH; Novartis) has a UK list price of £282,000 for a one-time infusion, and atidarsagene autotemcel (Libmeldy; Orchard Therapeutics) has a UK list price of £2.9m, making it the world’s most expensive medicine[4]. Although patient access schemes and managed access agreements offer discounts to the NHS, the costs are nonetheless exceptionally high, leaving commissioners and policymakers with difficult decisions concerning value for money, reimbursement and budgets[41].
Assessing value
Some ATMPs are evaluated via NICE’s highly specialised technologies workstream, while others in England have been recommended via the Cancer Drugs Fund[4]. As they have been recommended via different pathways, different value judgements will have been made in relation to other NHS services. For instance, an average gain of one year in perfect health (equivalent to one quality-adjusted life year), may be valued over ten times higher in patients who might benefit from ATMPs, compared with other patients. This may be justified on the basis that ATMPs are more likely to be one-off, curative treatments[42].
However, in many instances, a lack of long-term outcome data, including health-related quality of life and survival, and reliance on endpoints that may be poor surrogates for outcomes of importance to patients, present challenges to decision makers. Economic evaluations of ATMPs conducted to date have revealed a high level of uncertainty in cost-effectiveness estimates because of the lack of long-term outcome data[2].
Paying for ATMPs
At present, ATMPs are paid for via standard methods of reimbursement, which involves an upfront cost to the NHS irrespective of whether the treatment succeeds or fails. Alternative payment methods are being proposed that share the financial risks between the NHS and pharmaceutical companies and facilitate timely patient access while evidence matures[20]. They include annual payments over a defined period that are dependent on meeting an agreed threshold of treatment response, or rebates that are payable if a patient outcome is not achieved or maintained. These will become increasingly important as the number of ATMPs increases and clinical indications extend from rare to more common diseases.
Conclusion
ATMPs have already delivered exciting outcomes and promise to deliver more in the future. Our workforce must be suitably informed to meet ATMP challenges as the NHS embraces these medicines, which have the potential to be life-changing for many patients.
The authors collectively make up the Adopting New Technology sub-group of the Science and Research Committee, Royal Pharmaceutical Society
- 1Challenges with advanced therapy medicinal products and how to meet them. Nat Rev Drug Discov. 2010;9:195–201. doi:10.1038/nrd3052
- 2Lloyd‐Williams H, Hughes DA. A systematic review of economic evaluations of advanced therapy medicinal products. Br J Clin Pharmacol. 2020;87:2428–43. doi:10.1111/bcp.14275
- 3Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol. 2013;10:267–76. doi:10.1038/nrclinonc.2013.46
- 4Pinho-Gomes A-C, Cairns J. Evaluation of Advanced Therapy Medicinal Products by the National Institute for Health and Care Excellence (NICE): An Updated Review. PharmacoEconomics Open. 2021;6:147–67. doi:10.1007/s41669-021-00295-2
- 5Fumagalli F, Calbi V, Natali Sora MG, et al. Lentiviral haematopoietic stem-cell gene therapy for early-onset metachromatic leukodystrophy: long-term results from a non-randomised, open-label, phase 1/2 trial and expanded access. The Lancet. 2022;399:372–83. doi:10.1016/s0140-6736(21)02017-1
- 6Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med. 2020;382:1331–42. doi:10.1056/nejmoa1914347
- 7Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377:2531–44. doi:10.1056/nejmoa1707447
- 8Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378:439–48. doi:10.1056/nejmoa1709866
- 9Whitford W, Hawkins I, Glamuzina E, et al. Compound heterozygous SLC19A3 mutations further refine the critical promoter region for biotin-thiamine-responsive basal ganglia disease. Cold Spring Harb Mol Case Stud. 2017;3:a001909. doi:10.1101/mcs.a001909
- 10Andtbacka RHI, Collichio F, Harrington KJ, et al. Final analyses of OPTiM: a randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III–IV melanoma. j. immunotherapy cancer. 2019;7. doi:10.1186/s40425-019-0623-z
- 11Sharma B, Singh N. Salutary effect of NFκB inhibitor and folacin in hyperhomocysteinemia–hyperlipidemia induced vascular dementia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2012;38:207–15. doi:10.1016/j.pnpbp.2012.03.013
- 12Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65 -mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. The Lancet. 2017;390:849–60. doi:10.1016/s0140-6736(17)31868-8
- 13Stirnadel-Farrant H, Kudari M, Garman N, et al. Gene therapy in rare diseases: the benefits and challenges of developing a patient-centric registry for Strimvelis in ADA-SCID. Orphanet J Rare Dis. 2018;13. doi:10.1186/s13023-018-0791-9
- 14Eschen C, Kaps C, Widuchowski W, et al. Clinical outcome is significantly better with spheroid-based autologous chondrocyte implantation manufactured with more stringent cell culture criteria. Osteoarthritis and Cartilage Open. 2020;2:100033. doi:10.1016/j.ocarto.2020.100033
- 15Onasemnogene abeparvovec for treating spinal muscular atrophy. National Institute for Health and Care Excellence. 2021.https://www.nice.org.uk/guidance/hst15 (accessed Jul 2022).
- 16McEntee J. Horizon scanning for ATMPs. Specialist Pharmacy Service. 2020.www.sps.nhs.uk/articles/horizon-scanning-for-atmps/ (accessed Jul 2022).
- 17Advanced therapy medicinal products. Specialist Pharmacy Service. 2022.www.sps.nhs.uk/home/guidance/advanced-therapy-medicinal-products/ (accessed Jul 2022).
- 18Black A. Implementation of advanced therapy medicinal products: a UK pharmacist’s perspective. Cell Gene Therapy Insights. 2019;5:1237–46. doi:10.18609/cgti.2019.130
- 19Black A. Pharmacy Institutional Readiness for Marketed CAR-T Therapy: Guidance for Chief Pharmacists V4 (updated January 2020). Specialist Pharmacy Service. 2018.www.sps.nhs.uk/articles/pharmacy-institutional-readiness-for-marketed-car-t-therapy-guidance-for-chief-pharmacists/ (accessed Jul 2022).
- 20ATMPs being delivered in the UK. Advanced Therapy Treatment Centres. 2022.https://attc-143fd.kxcdn.com/wp-content/uploads/2022/03/Final-version-map-and-calendar-combined.pdf (accessed Jul 2022).
- 21Axicabtagene ciloleucel for treating diffuse large B-cell lymphoma and primary mediastinal large B-cell lymphoma after 2 or more systemic therapies. National Institute for Health and Care Excellence. 2019.https://www.nice.org.uk/guidance/ta559 (accessed Jul 2022).
- 22Tisagenlecleucel for treating relapsed or refractory diffuse large B-cell lymphoma after 2 or more systemic therapies. National Institute for Health and Care Excellence. 2019.https://www.nice.org.uk/guidance/ta567 (accessed Jul 2022).
- 23Onasemnogene abeparvovec for treating spinal muscular atrophy. National Institute for Health and Care Excellence. 2021.https://www.nice.org.uk/guidance/hst15 (accessed Jul 2022).
- 24Autologous anti-CD19-transduced CD3+ cells for treating relapsed or refractory mantle cell lymphoma. National Institute for Health and Care Excellence. 2021.https://www.nice.org.uk/guidance/ta677 (accessed Jul 2022).
- 25Talimogene laherparepvec for treating unresectable metastatic melanoma. National Institute for Health and Care Excellence. 2016.https://www.nice.org.uk/guidance/ta410 (accessed Jul 2022).
- 26Holoclar for treating limbal stem cell deficiency after eye burns. National Institute for Health and Care Excellence. 2017.https://www.nice.org.uk/guidance/ta467 (accessed Jul 2022).
- 27Voretigene neparvovec for treating inherited retinal dystrophies caused by RPE65 gene mutations. National Institute for Health and Care Excellence. 2019.https://www.nice.org.uk/guidance/hst11 (accessed Jul 2022).
- 28Autologous chondrocyte implantation using chondrosphere for treating symptomatic articular cartilage defects of the knee. National Institute for Health and Care Excellence. 2018. https://www.nice.org.uk/guidance/ta508 (accessed Jul 2022).
- 29Atidarsagene autotemcel for treating metachromatic leukodystrophy. National Institute for Health and Care Excellence. 2022.https://www.nice.org.uk/guidance/HST18/history (accessed Jul 2022).
- 30New roadmap to help speed up patient access to ATMP products. Association of the British Pharmacological Industry. 2021.https://www.abpi.org.uk/media/news/2021/december/new-roadmap-to-help-speed-up-patient-access-to-atmp-products/ (accessed Jul 2022).
- 31Guidelines on Good Manufacturing Practice specific to Advanced Therapy Medicinal Products. European Commission. 2017.https://health.ec.europa.eu/system/files/2017-11/2017_11_22_guidelines_gmp_for_atmps_0.pdf (accessed Jul 2022).
- 32Professional Guidance for the Procurement and Supply of Specials. Royal Pharmaceutical Society. 2015.https://www.rpharms.com/Portals/0/RPS%20document%20library/Open%20access/Support/toolkit/specials-professional-guidance.pdf (accessed Jul 2022).
- 33Black A. Out of Specification Advanced Therapy Medicinal Products – Guidance for Healthcare Organisations. Specialist Pharmacy Service. 2020.https://www.sps.nhs.uk/articles/out-of-specification-advanced-therapy-medicinal-products-guidance-for-healthcare-organisations/ (accessed Jul 2022).
- 34Barrett NA, Jones A, Whiteley C, et al. Management of long-term hypothyroidism: a potential marker of quality of medicines reconciliation in the intensive care unit†. International Journal of Pharmacy Practice. 2012;20:303–6. doi:10.1111/j.2042-7174.2012.00205.x
- 35Black A. Supportive Medicines for Adults Receiving Marketed CAR-T Cell Therapy. Specialist Pharmacy Service. 2022.www.sps.nhs.uk/articles/supportive-medicines-for-adults-receiving-marketed-car-t-cell-therapy/ (accessed Jul 2022).
- 36Black A. Diagnosis and Medical Management of Acute CAR-T Cell Toxicities in Adults. Specialist Pharmacy Service. 2020.https://www.sps.nhs.uk/articles/diagnosis-and-medical-management-of-acute-car-t-cell-toxicities-in-adults/ (accessed Jul 2022).
- 37NHS England strikes deal for ground breaking cancer treatment in a new European first. NHS England. 2018.https://www.england.nhs.uk/2018/10/nhs-england-strikes-deal-for-ground-breaking-cancer-treatment-in-a-new-european-first/#:~:text=News-,NHS%20England%20strikes%20deal%20for%20ground%20breaking,in%20a%20new%20European%20first&text=Adult%20cancer%20patients%20in%20England,executive%20Simon%20Stevens%20announced%20today (accessed Jul 2022).
- 38Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. j. immunotherapy cancer. 2018;6. doi:10.1186/s40425-018-0343-9
- 39Lanitis E, Coukos G, Irving M. All systems go: converging synthetic biology and combinatorial treatment for CAR-T cell therapy. Current Opinion in Biotechnology. 2020;65:75–87. doi:10.1016/j.copbio.2020.01.009
- 40Advanced Therapies NHS Readiness Toolkit. Advanced Therapy Treatment Centres. 2020.https://www.theattcnetwork.co.uk/advanced-therapies-nhs-readiness-toolkit (accessed Jul 2022).
- 41Champion AR, Lewis S, Davies S, et al. Managing access to advanced therapy medicinal products: Challenges for NHS Wales. Br J Clin Pharmacol. 2020;87:2444–9. doi:10.1111/bcp.14286
- 42Jönsson B, Hampson G, Michaels J, et al. Advanced therapy medicinal products and health technology assessment principles and practices for value-based and sustainable healthcare. Eur J Health Econ. 2018;20:427–38. doi:10.1007/s10198-018-1007-x


1 comment