JOHN BIRDSALL SOCIAL ISSUES PHOTO LIBRARY/SCIENCE PHOTO LIBRARY
After the reading this article, you should be able to:
- Understand how the National Institute for Health and Care Excellence guidance has changed and how this affects the management of chronic kidney disease, including renal anaemia;
- Detail the available treatments for anaemia in patients with chronic kidney disease, including intravenous iron preparations and erythropoiesis-stimulating agents;
- Understand that the emergence of hypoxia-inducible factor prolyl-hydroxylase inhibitors offers an alternative to erythropoiesis-stimulating agents in the management of renal anaemia.
Chronic kidney disease is the progressive and usually irreversible decline in renal function over time, particularly associated with ageing, hypertension, type 1 and type 2 diabetes mellitus, and a variety of other contributory factors. It is classified by cause, impairment of estimated glomerular filtration rate (eGFR), and degree of albuminuria[1]. New, comprehensive guidance from the National Institute for Health and Care Excellence on the assessment and management of chronic kidney disease (CKD) was published in August 2021[2]. This replaces the earlier 2014 guideline and incorporates the former guidance on hyperphosphataemia from 2013 and anaemia of CKD (ACKD) from 2015 into a single document. ACKD is associated with worsening decline in renal function and has been covered in ‘Diagnosis and management of anaemia in adults with chronic kidney disease’[3].
Changes to the NICE CKD guideline may appear subtle, but pharmacists should be aware of amendments to the management of iron and injected erythropoiesis-stimulating agents (ESAs). More significantly, in August 2021, the first hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI), roxadustat, was licensed in the UK and EU, representing the most significant advancement in the management of renal anaemia in a decade[4]. In July 2022, NICE published a technology appraisal approving and supporting its use in the NHS in England and Wales; decisions are awaited in Scotland and Northern Ireland[5].
This article will cover changes to practice brought about by the updated NICE CKD guideline, as well as recent advances in ACKD treatment using HIF-PHIs[2].
Management of oral and intravenous iron
Adequate iron availability is essential for the maintenance of erythropoiesis and the correction of anaemia. NICE recommends the use of two markers, the percentage of hypochromic red blood cells, which should be below 6%, or, alternatively, reticulocyte haemoglobin content, which should be ≥29 picograms, unless serum ferritin is greater than 800microgram/L[2]. Unfortunately, these tests are still not universally available throughout the NHS (and cannot be used in patients with thalassaemia or thalassaemia trait); therefore, the traditional tests of serum ferritin and percentage transferrin saturation are more frequently used.
Serum ferritin reflects (but is only a surrogate for) liver ferritin, the second most important site of iron storage in the body. A failure to maintain a serum ferritin level of 100microgram/L or more is considered to represent absolute iron deficiency. However, serum ferritin can be elevated in infections and inflammatory conditions without iron replete status[6]. Ferritin should not be allowed to rise above 800mcg/L (with action taken at 500mcg/L to avoid the development of iron overload)[2].
Transferrin saturation should be 20% or more to avoid a functional iron deficiency, in which the iron-transporting protein transferrin cannot immediately make available sufficient iron to the bone marrow for erythropoiesis, even in the presence of adequate liver stores[2].
Both serum ferritin and transferrin saturation measurements are required to be assessed together to ensure that there is sufficient iron immediately available for erythropoiesis (no functional deficiency) and no concern about adequate further stores to call on (absolute iron deficiency).
Oral iron may have a place in the management of iron deficiency in anaemia in CKD, but poor tolerability and low absorption mean that it may not provide sufficient benefit. NICE recommends that intravenous iron (IVI) should be offered if oral therapy is not tolerated or ineffective within three months[2]. If patients start haemodialysis, (where blood losses are higher) or if they start erythropoiesis-stimulating therapy (where iron demand will rise as erythropoiesis increases again) NICE states that IVI is to be offered, unless contraindicated or the patient refuses after an informed discussion of its risks and benefits.
Modern IVI preparations are much safer than earlier iron therapies and increasingly used in patients with other medical conditions, such as gastrointestinal diseases with reduced iron absorption, and symptomatic heart failure with iron deficiency, where there are both guidelines from the European Society of Cardiology that support this and a strong evidence base of morbidity/mortality benefit[7]. They are also increasingly used in surgery and severe anaemias to minimise transfusion demand, supported by NICE guidance[8,9].
Calculating iron dose
Calculations for iron dosage are based on the fact that there is 3.34mg of iron in every 1g of haemoglobin, and formulae, such as that of Garzoni, which assumes around 150mg of iron is needed to raise the haemoglobin of a typical adult by 10g/L[10,11]. An adult with a need to raise serum haemoglobin by 30g/L will need around 500mg of iron plus about the same to correct depleted stores: that is, about 1,000mg of iron. In haemodialysis patients, ongoing blood losses may require more than 2,000–3,000mg of iron annually to maintain iron replete status[12].
The most common preparations and their restrictions have been described elsewhere but essentially can be divided into two types[3]. Firstly, there are IVI preparations that are licensed for low-dose, high-frequency use, such as ferric derisomaltose and iron sucrose. These IVIs are less expensive than the alternative high-dose, low-frequency (HDLF) preparations but are limited by their licences to a maximum single dose of 200mg of iron[13,14]. This regimen is not inconvenient for most haemodialysis patients, where repeated IV access is readily available during frequent haemodialysis sessions, but in non-haemodialysing CKD patients and non-renal patients, several clinic visits will be necessary to infuse the full dose required. HDLF preparations are more expensive but allow larger doses — potentially the full amount required — on a single infusion. They include ferric derisomaltose and ferric carboxymaltose[15,16]. These can be given in single doses of up to 20mg/kg (maximum single administration 1g for ferric carboxymaltose) making them more convenient in non-dialysing inpatients, outpatients, pre-dialysis and peritoneal dialysis patients. In addition, since the Medicines and Healthcare products Regulatory Agency (MHRA) prohibits administration of IVI outside locations with resuscitation facilities, HDLF iron infusions are also useful for home-haemodialysing patients (but only using ferric derisomaltose as ferric carboxymaltose has a dose restriction of 200mg in haemodialysis-dependent patients)[16].
The PIVOTAL trial (Proactive IV Iron Therapy in Haemodialysis Patients), a multicentre, open-label trial with blinded end-point evaluation, the results of which were published in 2019, followed more than 2,100 haemodialysis patients receiving iron sucrose for more than 2 years[12]. One arm received iron sucrose reactively: 0–400mg monthly when serum ferritin fell below 200microgram/L or transferrin saturation below 20%. Patients in the other arm proactively received 600mg in the first month, then 400mg monthly (unless ferritin exceeded 700mcg/L or transferrin saturation exceeded 40%). In practice, the median monthly doses received were 264mg in the proactive high-dose patients versus 145mg in the reactive group. Although serum ferritin and transferrin saturation were significantly higher in the group receiving higher doses of iron, serum haemoglobins achieved were similar because of ESA adjustment. However, in the proactively treated arm, there was a reduction in the composite end-point of non-fatal myocardial infarction or stroke, admissions for heart failure, or death without increasing adverse drug reactions or infections. In addition, ESA doses required to maintain haemoglobin levels were about a fifth lower, more than compensating for the increased iron costs.
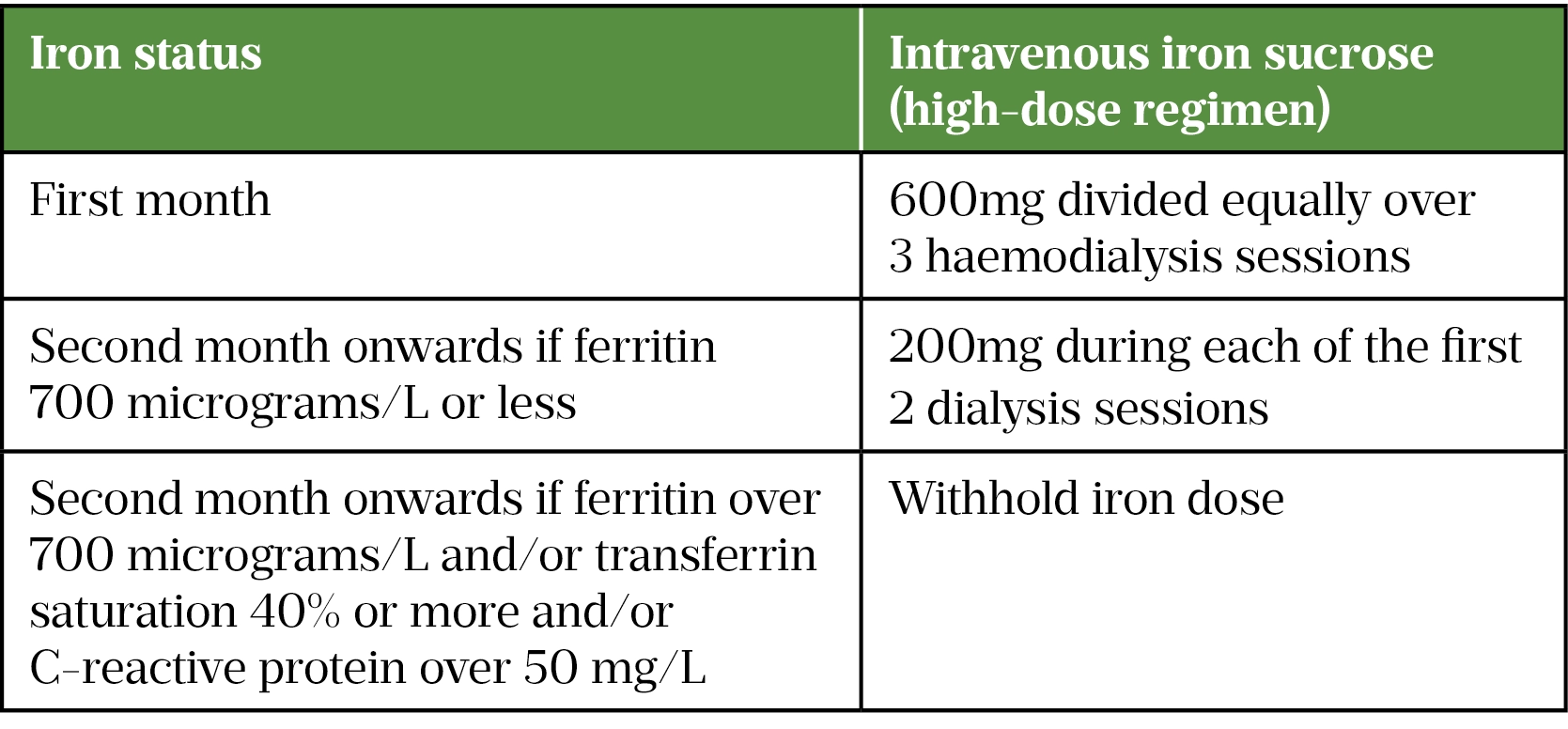
The UK Renal Registry publishes data showing considerable variation between UK haemodialysis centres in the number of patients achieving an in-range serum haemoglobin (100–120g/L) and those not achieving a minimum serum ferritin (≥ 100microgram/L); however, information on ESA dosing is incomplete and absent for iron dosing[17]. This most likely reflects the greater ease of assembling computerised laboratory results compared to prescription data for large numbers of patients. It is certainly possible that mean iron dosing schedules exceed the PIVOTAL higher dose schedule already in local units. However, in the light of PIVOTAL, NICE updated its recommendations on the use of IVI in haemodialysis patients to stress the need to use higher doses of IVI, especially on initiation (see Table)[2]. While the evidence supporting this recommendation was derived from iron sucrose, the results were likely not dependent on the type of IVI used and local guidelines may have to adapt to local purchasing practices. In addition, the PIVOTAL trial dosing adopted in the NICE recommendations may not be the most practical schedule in local units. It is important to note that the recommendations only apply to haemodialysis patients, but the principle of increasing iron doses to achieve optimal patient benefits may be applicable in all patients receiving IVI. The NICE guidance has always stressed the need to start iron before or with ESAs to ensure adequate iron availability for erythropoiesis[2].
Updated recommendations for the management of CKD
The differences between the 2015 and 2021 NICE recommendations for the assessment and management of CKD are subtle but significant. Standard creatinine-based eGFR and albumin-creatinine ratio (ACR) are still recommended for the assessment of CKD, being both cheaper and at least as accurate (the 2015 guideline had recommended moving to estimations based on the protein cystatin-C)[2,18]. In addition, while caution is needed, patients of African or Afro-Caribbean origin should no longer have a correction factor applied when calculating eGFR as it has not been proven to improve usefulness or accuracy, especially when considering the variety of body types within a single ethnic group[2,18].
Once an eGFR has been established (repeating after 2 weeks, emphasising no meat for 12 hours before the test for a first estimation of <60mL/minute/1.73m2), primary care clinicians, such as GPs, practice nurses and pharmacists, should additionally no longer rely solely on the result when deciding who to refer to specialist nephrologists. Instead, the 4-variable kidney failure risk equation (4v-KFRE) is recommended to predict and refer adults with a 5% risk of progression to end-stage kidney failure within five years. 4v-KFRE uses patient age, sex, eGFR and ACR to estimate the risk of progressing to a need for renal replacement therapy — whether this is dialysis or transplant — and is easily generated with online calculators[19]. ACR urinary albumin–creatinine ratio is considered a prognostic marker of poor prognosis and levels above 3mg/mmol are considered particularly clinically significant, even if eGFR has not yet decreased significantly.
Risk prediction using only four variables might seem simplistic, given the well-recognised increased risk of CKD progression in patients with hypertension, diabetes, other cardiovascular diseases, who smoke, or with African, Afro-Caribbean or Asian heritage. However, many of these variables will have effectively already been incorporated into either ACR or their eGFR compared to that expected for their age. In addition, the guidelines continue to suggest that a rapidly falling eGFR, an ACR of more than 70mg/mmol (unless known to be caused by diabetes and optimally treated) or more than 30mg/mmol with haematuria, refractory hypertension, genetic diseases or suspected renal artery stenosis all require prompt referral[2].
Serum glucose co-transporter-2 inhibitors
Another set of changes in the NICE CKD 2021 guidance, incorporated from the recent update to the type 2 diabetes mellitus (T2DM) guideline, relate to the use of serum glucose co-transporter-2 (SGLT-2) inhibitors, including dapagliflozin, canagliflozin, empagliflozin, and ertugliflozin, now licensed for T2DM[20–24]. SGLT-2 inhibitors are used to treat T2DM by promoting glucose excretion into the urine, leading to an induced glycosuria[25]. NICE now recommends that after optimising standard angiotensin-converting enzyme inhibitor/angiotensin receptor blocker therapy, and where not contra-indicated by comorbidities or the e-GFR limits in the summary of product characteristics (SPC), SGLT-2 inhibitors should be offered to T2DM patients with an ACR of >30mg/mmol, and at least considered if ACR is 3–30mcg/mmol.
SGLT-2 inhibitors also have benefits in non-diabetic patients. Empagliflozin and dapagliflozin are licensed and recommended as part of the treatment of chronic heart failure, where they have been shown to reduce cardiovascular mortality[21,23,26,27]. In addition, they are also known to reduce CKD progression, proteinuria, blood pressure and weight — at time of writing, dapagliflozin has been licensed for this[21,28,29]. In guidelines produced for the management of CKD, the UK Kidney Association (UKKA) describes these medicines as a “paradigm shift”[30]. In March 2022, NICE published the technology appraisal TA775, supporting the use of dapagliflozin for CKD (25–75mL/min) in patients who either have T2DM or an ACR of more than 22.6mg/mmol[31]. Given the large number of NHS patients with albuminuric CKD, with or without diabetes, this technology appraisal was necessary before trusts and GPs could adopt the UKKA recommendations[32].
SGLT-2 inhibitors are not without their own risks, such as diabetic ketoacidosis (especially if used concurrently with other hypoglycaemic agents), acute kidney injury secondary to dehydration, urinary tract infections, Fournier’s gangrene (affecting the genitourinary tract and often secondary to glycosuria) and increased fracture/amputation risks[21,30,33,34]. For this reason, UKKA recommends very careful patient selection, monitoring and counselling, and has prepared draft patient information leaflets for both diabetic and non-diabetic patients, which will help prescribers and pharmacists counsel to minimise risks[30].
Erythropoiesis-stimulating agents
ESAs are indicated, once ACKD is determined owing to low levels of endogenous erythropoietin. Levels are rarely taken, rather a reduced eGFR and exclusion of other causes, such as iron or folate deficiency, gastrointestinal bleeds or malignancy will indicate the need for ESA therapy. The original ESAs designed for either subcutaneous or IV (during haemodialysis) administration (epoetin alfa, epoetin beta, and epoetin zeta) have essentially the same structure as erythropoietin. The analogues darbepoetin and methoxy polyethylene glycol-epoetin beta were subsequently developed to enable a reduced dosing frequency[3,35–39]. In addition, the latter enable IV dosing without loss of dosing efficacy compared to subcutaneous administration, thus allowing less painful intravenous administration in haemodialysis patients without additional costs[38,39]. Additional detail on ESA subtypes and choice can be found here. NICE acknowledges that little evidence exists to support that one preparation achieves better outcomes than another; therefore, factors such as drug availability, patient preference and dialysis status should affect choice[2].
In practice, most patients on in-centre (hospital or dialysis unit) haemodialysis are supplied their treatment from hospital/dialysis unit stock. Other patients will self-administer ESA or have it administered to them by community-based staff; this is either individually dispensed or through a hospital-led homecare scheme. The increasing discounts available for large, regional volume-based contracts means that most patients in an area will be on the same ESA, selected on both acquisition price and local preferences.
Previous NICE guidance did not advocate for target haemoglobin levels of >120g/L, recommending 115g/L to prevent exceeding targets, and preventing haemoglobin from rising by more than 20g/L each month; this remains unchanged in the 2021 NICE guideline[2]. This is because of the lack of clinical benefit and increasing cardiovascular risks, particularly stroke risk, of ESA-driven high haemoglobin levels, highlighted by the MHRA[2,40]. Lower target haemoglobin levels are preferred, possibly even below the target range, if high ESA doses are required to achieve targets or increasing doses are ineffective at increasing haemoglobin. These guidelines are informed by results from trials using epoetin alfa (CHOIR), epoetin beta (CREATE), and darbepoetin alfa (TREAT), so can be presumed to be a class effect[41–43]. In all of these studies, required ESA doses, costs and increasing cardiovascular mortality did not justify increasing haemoglobin levels. Other than reemphasising these points, the new NICE guideline does not substantially change previous recommendations regarding ESA therapy use in adults.
Hypoxia-inducible factor prolyl hydroxylase inhibitors
Recombinant synthetic erythropoietin was first licensed for clinical use as epoetin in 1989, transforming the management of renal anaemia[44]. With the subsequent variants detailed above, parenteral ESAs have become the standard therapy for haemoglobin restoration. However, in a manner similar to insulins, their great disadvantage is that, no matter how much the protein is modified, parenteral therapy is still needed, along with cold chain security of the product.
Pharmacology
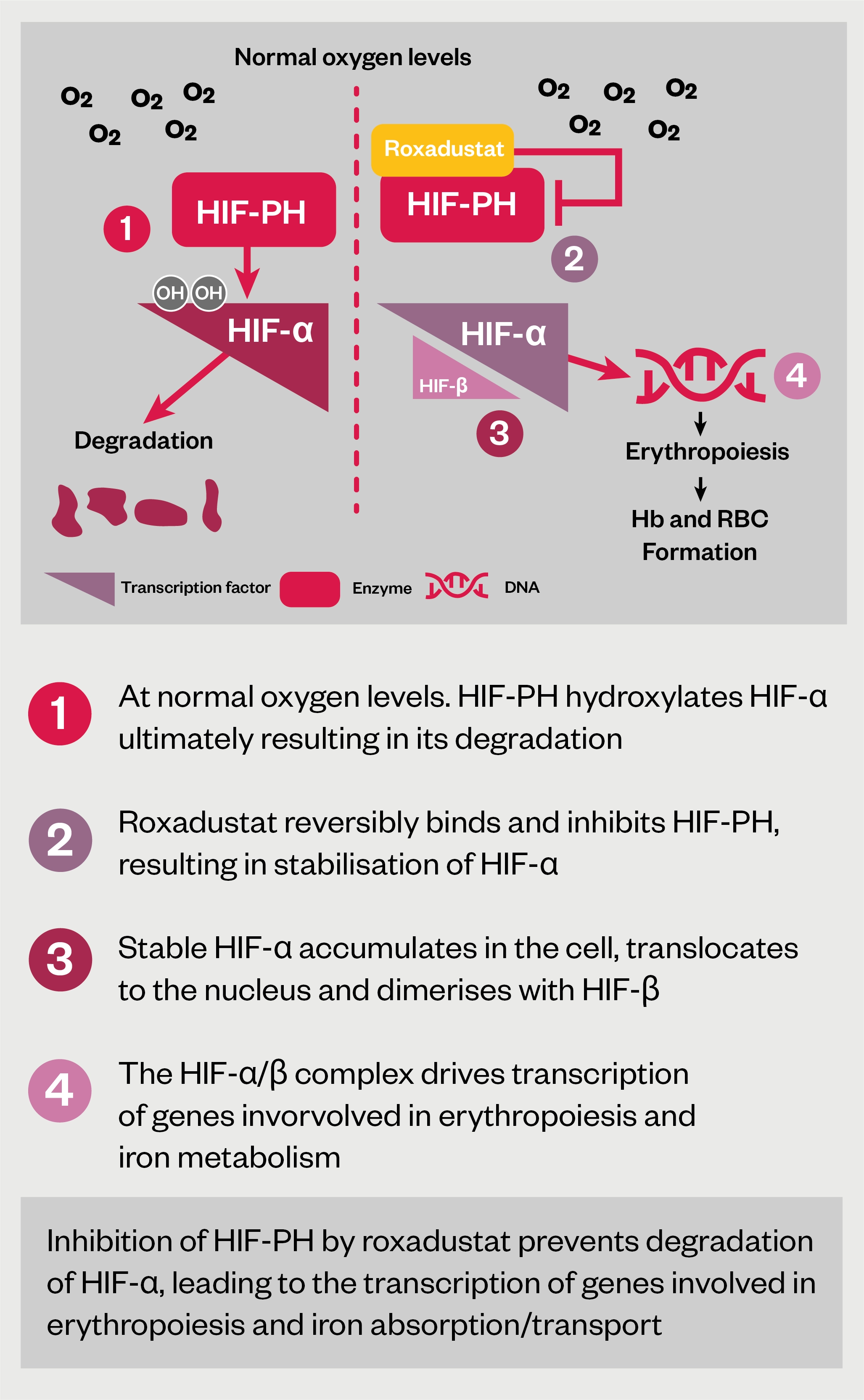
The mechanism of action of HIF-PHIs can be seen in Figure 1[45–47].
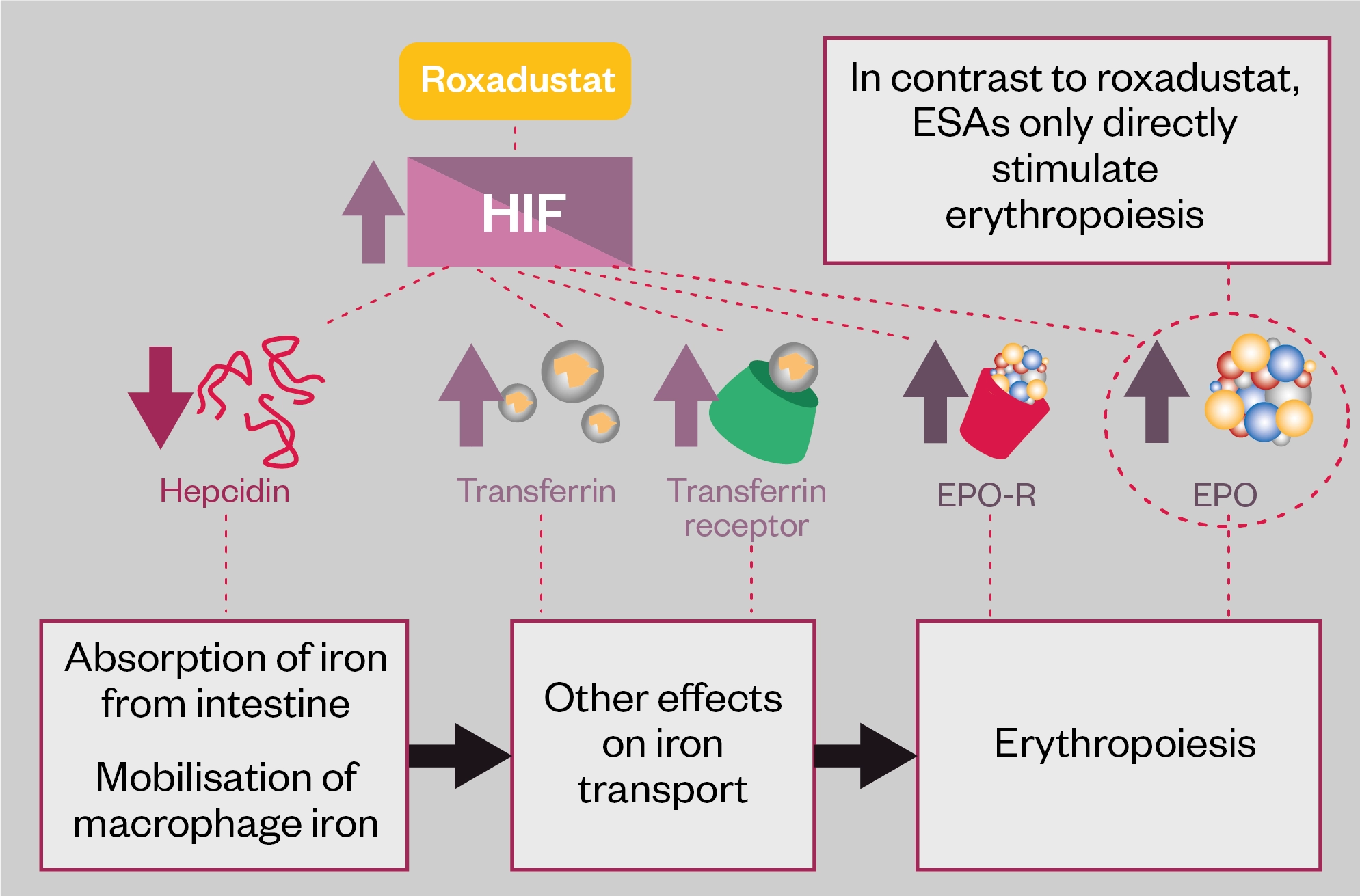
Hepcidin is a liver-produced peptide hormone that is made in response to infection and inflammation, as well as iron excess, and is increased when its renal clearance is reduced. Increased hepcidin production leads to reduced iron absorption, mobilisation and recycling in the body. Anaemia, hypoxia and increasing erythropoiesis all suppress hepcidin and so increase iron availability[45–47]. HIF therefore also acts indirectly to reduce hepcidin levels and increase iron availability for erythropoiesis.
In short, by reducing breakdown by hydroxylation, HIF-PHIs increase HIF levels and natural erythropoietin in a similar manner to hypoxia, and by less clear and indirect mechanisms, suppress hepcidin, increasing both iron availability and mobilisation, resulting in increasing serum haemoglobin[45–47]. This seemingly leads to erythropoiesis with much lower levels of erythropoietin than the equivalent effects that administration of synthetic ESAs would generate, and possibly a reduced need for IV iron infusions[47]. HIF-PHIs may even be able to stimulate erythropoietin production via stimulation of minor sites of production in the liver in anephric patients[47].
Roxadustat
HIF-PHIs are now available for use. The option of an oral alternative to ESAs represents an advance in the management of renal anaemia, especially for patients not on unit-based haemodialysis, who would otherwise have to be supplied with ESAs at home, inject themselves or arrange for professionals to do so, and maintain cold chain. Roxadustat was licensed in the both the UK and Europe in August 2021[4]. In the United States, however, the Federal Drugs Administration is requesting additional safety data and has not approved roxadustat at the time of publication[48].
Roxadustat is the first in its class and a series of other HIF-PHIs are in development, some of which are licensed in Asian countries and may eventually be launched in the UK. Phase III trial results have shown rising haemoglobin with daprodustat, vadadustat, enarodustat, and molidustat, with other agents under development[47].
Roxadustat is licensed at a starting dose of 70mg three times per week, rising to 100mg three times per week in patients who weigh 100kg and above. The SPC also contains details of how to convert patients from standard ESA therapies, although this should not be done for stable dialysis patients without good reason[4]. The dose can then be titrated in a similar manner to ESAs — i.e. to maintain a target haemoglobin of 100–120g/L, increasing by no more than 20g/L in any month.
As a result, monthly monitoring of haemoglobin is still required, and these haemoglobin levels are related to a titration schedule for roxadustat in the SPC. The maximum dose is 3mg/kg three times per week or 300mg (non-dialysis patients)/400mg (dialysis patients). As with ESAs, prescribers should ensure iron stores are still adequate. The SPC aims for a target haemoglobin of 100–120g/L, which matches the NICE standards when using traditional ESAs[4].
Unlike traditional ESAs, use in the third trimester of pregnancy or in breastfeeding is contraindicated. Use of roxadustat in severe hepatic impairment (Child-Pugh class C) is also not recommended. Finally, because of reduced absorption, phosphate binders and metallic salts, such as calcium and magnesium, should be withheld before roxadustat use for at least an hour, and care should be taken in concurrent use with simvastatin as levels are substantially increased[4].
Clinical efficacy for roxadustat in achieving target haemoglobin levels (100–120g/L) and overall safety were similar to outcomes using ESA therapy in clinical trials[5,45–47]. However, the NICE technology appraisal observes that only one good trial comparing roxadustat to ESAs (using darbepoetin alfa) as compared to placebo in non-dialysis patients (the approved group in the technology appraisal) currently exists[5]. Hypertension, vascular access thrombosis, diarrhoea, peripheral oedema, hyperkalaemia and nausea are very commonly reported (>10% of patients). Adverse drug reactions (ADRs) classified as serious that were common (>1% of patients) included sepsis, hyperkalaemia, hypertension and deep vein thrombosis. The SPC should be consulted for a full list and, as a Black Triangle drug, a Yellow Card should be sent to the MHRA for all suspected adverse events.
The list price of roxadustat is £2,700 annually for the standard starting dose — making the cost comparable with the list price of ESAs — and between £800 and £15,000 annually for maintenance, depending on the dose[49]. However, as previously mentioned, most NHS providers contract for ESAs in bulk at fractions of the list price, meaning that, without a discount, roxadustat would struggle to gain market share against ESAs.
In the final NICE technology appraisal, roxadustat was approved by NICE in non-dialysis patients (at the time of initiation) in CKD stages 3–5, with an eGFR of less than 60mL/minute, who are iron replete. In addition, the roxadustat has to be provided under a confidential commercial arrangement with the NHS. These arrangements allow the NHS to introduce new therapies at a cost agreed with the manufacturer of an expensive drug, without changing the published and internationally visible NHS list price[50]. It seems reasonable to presume that the manufacturer has had to reduce the effective price of roxadustat to secure approval for use in the NHS. The advantages to patients and trusts of having an approved NICE technology appraisal is that NHS providers in England have to make the drug available (where physicians feel it appropriate) within three months of the appraisal being published, (two months in Wales) and purchasing authorities are required to fund it[5].
The current NICE technology appraisal has excluded dialysis patients from approval, but roxadustat offers no advantages over ESAs in efficacy, safety or ease of administration, at least in haemodialysis patients[5]. Many patients currently on ESAs receive them in the community. For example, patients with non-dialysis-dependent CKD (including post-transplant) and conservative management patients (the latter group have reached near end-stage kidney failure but, owing to age or comorbidities, starting dialysis or listing for transplant may not be in their best interests)[51]. In these cohorts, an oral therapy offers real advantages over an injectable product requiring cold-chain supply and storage, especially in terms of patient convenience.
Depending on how ESAs are currently supplied, some of the increased costs of using roxadustat, if any, may be offset. These include the costs of refrigeration of ESAs and overall storage space required if dispensed from hospitals, and service/delivery charges if a homecare provider is used. There are also savings elsewhere in the NHS in community nursing time if age or comorbidities mean self-injection of ESAs is not possible, although longer acting ESAs, such as darbepoetin or methoxy polyethylene glycol-epoetin beta that require fewer administrations should already be preferred in this population[38,39].
Conclusion
The updated NICE CKD guideline and the subsequent licencing of dapagliflozin and roxadustat, with TAs to encourage and fund their use, represent changes to the management of CKD that pharmacists should be aware of.
NICE has managed cost issues and made roxadustat available to non-dialysis patients — the first oral alternative to traditional injectable ESAs, with broadly similar efficacy and safety profiles. It should not be forgotten that there is substantially less trial and clinical experience with oral HIF-PHIs, or that there will still be a need for monthly blood tests, specialist team prescribing, and monitoring for safe and effective care[4,5].
- 1KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney International Supplements. 2012.https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-Anemia-Guideline-English.pdf (accessed Aug 2022).
- 2Chronic kidney disease: assessment and management. National Institute for Health and Care Excellence. 2021.https://www.nice.org.uk/guidance/ng203 (accessed Aug 2022).
- 3Brown C, Jackson D. Diagnosis and management of anaemia in adults with chronic kidney disease. The Pharmaceutical Journal. 2018.https://pharmaceutical-journal.com/article/ld/diagnosis-and-management-of-anaemia-in-adults-with-chronic-kidney-disease (accessed Aug 2022).
- 4Evrenzo (roxadustat) film coated tablets. Electric medicines compendium. 2021.https://www.medicines.org.uk/emc/product/12835/smpc#gref (accessed Aug 2022).
- 5Roxadustat for treating symptomatic anaemia in chronic kidney disease. National Institute for Health and Care Excellence. 2022.https://www.nice.org.uk/guidance/TA807 (accessed Aug 2022).
- 6KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International Supplements. 2013.https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf (accessed Aug 2022).
- 7McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. European Heart Journal. 2021;42:3599–726. doi:10.1093/eurheartj/ehab368
- 8Blood transfusion. National Institute for Health and Care Excellence. 2015.https://www.nice.org.uk/guidance/ng24 (accessed Aug 2022).
- 9Perioperative care in adults. National Institute for Health and Care Excellence. 2020.https://www.nice.org.uk/guidance/ng180 (accessed Aug 2022).
- 10Schrier S. So you know how to treat iron deficiency anemia. Blood 2015;126:1971. doi:10.1182/blood-2015-09-666511
- 11Koch T, Myers J, Goodnough L. Intravenous Iron Therapy in Patients with Iron Deficiency Anemia: Dosing Considerations. Anemia 2015;2015:763576. doi:10.1155/2015/763576
- 12Macdougall IC, White C, Anker SD, et al. Intravenous Iron in Patients Undergoing Maintenance Hemodialysis. N Engl J Med. 2019;380:447–58. doi:10.1056/nejmoa1810742
- 13Diafer 50 mg/ml solution for injection. Electronic medicines compendium. 2022.https://www.medicines.org.uk/emc/product/5324 (accessed Jul 2022).
- 14Venofer (iron sucrose) 20 mg iron / ml, solution for injection or concentrate for solution for infusion. Electronic medicines compendium. 2021.https://www.medicines.org.uk/emc/product/5911 (accessed Jul 2022).
- 15Monofer 100mg/ml solution for injection/infusion. Electronic medicines compendium. 2022.https://www.medicines.org.uk/emc/product/5676 (accessed Jul 2022).
- 16Ferinject (ferric carboxymaltose). Electronic medicines compendium. 2022.https://www.medicines.org.uk/emc/product/5910 (accessed Jul 2022).
- 17UK Renal Registry 23rd Annual Report. The Renal Association. 2021.https://ukkidney.org/sites/renal.org/files/publication/file-attachments/23rd_UKRR_ANNUAL_REPORT_0.pdf (accessed Jul 2022).
- 18Wilkinson E. NICE CKD update includes new referral rules and changes to ethnicity calculations. Pulse. 2021.https://www.pulsetoday.co.uk/news/clinical-areas/renal-medicine-urology/nice-ckd-update-includes-new-referral-rules-and-changes-to-ethnicity-calculations/ (accessed Aug 2022).
- 19Kidney Failure Risk Equation (4 Variable). QxMD. 2015.https://qxmd.com/calculate/calculator_308/kidney-failure-risk-equation-4-variable (accessed Aug 2022).
- 20Type 2 diabetes in adults: management. National Institute for Health and Care Excellence. 2022.https://www.nice.org.uk/guidance/ng28 (accessed Aug 2022).
- 21Forxiga 10 mg film-coated tablets. Electronic medicines compendium. 2022.https://www.medicines.org.uk/emc/product/7607/smpc (accessed Aug 2022).
- 22Invokana 100 mg film-coated tablets. Electronic medicines compendium. 2021.https://www.medicines.org.uk/emc/product/8855/smpc (accessed Aug 2022).
- 23Jardiance 10 mg film-coated tablets. Electronic medicines compendium. 2022.https://www.medicines.org.uk/emc/product/5441/smpc (accessed Aug 2022).
- 24Steglatro 5 mg Film-Coated Tablets. Electronic medicines compendium. 2022.https://www.medicines.org.uk/emc/product/9803/smpc (accessed Aug 2022).
- 25Hsia D, Grove O, Cefalu W. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes 2017;24:73–9. doi:10.1097/MED.0000000000000311
- 26Dapagliflozin for treating chronic heart failure with reduced ejection fraction. National Institute for Health and Care Excellence. 2021.https://www.nice.org.uk/guidance/ta679 (accessed Aug 2022).
- 27Empagliflozin for treating chronic heart failure with reduced ejection fraction. National Institute for Health and Care Excellence. 2022.https://www.nice.org.uk/guidance/ta773 (accessed Aug 2022).
- 28Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383:1436–46. doi:10.1056/nejmoa2024816
- 29Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380:2295–306. doi:10.1056/nejmoa1811744
- 30UK Kidney Association Clinical Practice Guideline: Sodium-Glucose Co-transporter-2 (SGLT-2) Inhibition in Adults with Kidney Disease. UK Kidney Association. 2021.https://ukkidney.org/sites/renal.org/files/UKKA%20guideline_SGLT2i%20in%20adults%20with%20kidney%20disease%20v1%2020.10.21.pdf (accessed Aug 2022).
- 31Dapagliflozin for treating chronic kidney disease. National Institute for Health and Care Excellence. 2022.https://www.nice.org.uk/guidance/ta775 (accessed Aug 2022).
- 32Dapagliflozin: Medicinal forms. National Institute for Health and Care Excellence. 2022.https://bnf.nice.org.uk/drugs/dapagliflozin/medicinal-forms/ (accessed Aug 2022).
- 33SGLT2 inhibitors: updated advice on the risk of diabetic ketoacidosis. Medicines and Healthcare products Regulatory Agency. 2016.https://www.gov.uk/drug-safety-update/sglt2-inhibitors-updated-advice-on-the-risk-of-diabetic-ketoacidosis (accessed Aug 2022).
- 34SGLT2 inhibitors: reports of Fournier’s gangrene (necrotising fasciitis of the genitalia or perineum). Medicines and Healthcare products Regulatory Agency. 2019.https://www.gov.uk/drug-safety-update/sglt2-inhibitors-reports-of-fournier-s-gangrene-necrotising-fasciitis-of-the-genitalia-or-perineum (accessed Aug 2022).
- 35Eprex 40,000 IU/ml solution for injection in pre-filled syringe. Electronic medicines compendium. 2021.https://www.medicines.org.uk/emc/product/1193/smpc#gref (accessed Aug 2022).
- 36NeoRecormon 10,000 IU solution for injection in pre-filled syringe. Electronic medicines compendium. 2021.https://www.medicines.org.uk/emc/product/10496/smpc# (accessed Aug 2022).
- 37Retacrit 1 000 IU/0.3 ml solution for injection in pre-filled syringe. Electronic medicines compendium. 2022.https://www.medicines.org.uk/emc/product/6413/smpc# (accessed Aug 2022).
- 38Aranesp solution for injection in pre-filled pen (SureClick). Electronic medicines compendium. 2022.https://www.medicines.org.uk/emc/product/6958/smpc# (accessed Aug 2022).
- 39Mircera solution for injection in pre-filled syringe. Electronic medicines compendium. 2021.https://www.medicines.org.uk/emc/product/360 (accessed Aug 2022).
- 40Recombinant human erythropoietins: new advice for prescribing. Medicines and Healthcare products Regulatory Agency. 2014.https://www.gov.uk/drug-safety-update/recombinant-human-erythropoietins-new-advice-for-prescribing (accessed Aug 2022).
- 41Singh AK, Szczech L, Tang KL, et al. Correction of Anemia with Epoetin Alfa in Chronic Kidney Disease. N Engl J Med. 2006;355:2085–98. doi:10.1056/nejmoa065485
- 42Drüeke TB, Locatelli F, Clyne N, et al. Normalization of Hemoglobin Level in Patients with Chronic Kidney Disease and Anemia. N Engl J Med. 2006;355:2071–84. doi:10.1056/nejmoa062276
- 43Pfeffer MA, Burdmann EA, Chen C-Y, et al. A Trial of Darbepoetin Alfa in Type 2 Diabetes and Chronic Kidney Disease. N Engl J Med. 2009;361:2019–32. doi:10.1056/nejmoa0907845
- 44Kalantar-Zadeh K. History of Erythropoiesis-Stimulating Agents, the Development of Biosimilars, and the Future of Anemia Treatment in Nephrology. Am J Nephrol 2017;45:235–47. doi:10.1159/000455387
- 45Locatelli F, Fishbane S, Block GA, et al. Targeting Hypoxia-Inducible Factors for the Treatment of Anemia in Chronic Kidney Disease Patients. Am J Nephrol. 2017;45:187–99. doi:10.1159/000455166
- 46Hirota K. Special Issue: Hypoxia-Inducible Factors: Regulation and Therapeutic Potential. Biomedicines. 2021;9:1768. doi:10.3390/biomedicines9121768
- 47Haase V. Hypoxia-inducible factor–prolyl hydroxylase inhibitors in the treatment of anemia of chronic kidney disease. Kidney International Supplements. 2021.https://www.haaselab.org/wp-content/uploads/2021/03/KISU_anemia_HIF_PHI_Haase.pdf (accessed Aug 2022).
- 48FDA declines to approve FibroGen’s roxadustat for anaemia of CKD. Pharmaceutical Technology. 2021.https://www.pharmaceutical-technology.com/news/fda-fibrogen-roxadustat-anaemia/ (accessed Aug 2022).
- 49Roxadustat: Medicinal forms. National Institute for Health and Care Excellence. 2022.https://bnf.nice.org.uk/drugs/roxadustat/medicinal-forms/ (accessed Aug 2022).
- 50NHS commercial framework for new medicines. NHS. 2022.https://www.england.nhs.uk/wp-content/uploads/2021/02/B1688-nhs-commercial-framework-for-new-medicines-june-22.pdf (accessed Aug 2022).
- 51Conservative Management for Kidney Failure. National Institute of Diabetes and Digestive and Kidney Diseases. 2018.https://www.niddk.nih.gov/health-information/kidney-disease/kidney-failure/conservative-management#:~:text=choose%20conservative%20management%3F-,What%20is%20conservative%20management%3F,kidney%20failure%20will%20be%20treated (accessed Aug 2022).
You might also be interested in…
EMA recommends treatment for rare kidney condition
