Alamy
Hepatitis E virus (HEV) is the most common cause of acute hepatitis in the UK[1]
. Around 20 million HEV infections occur annually worldwide, with more than 3 million symptomatic cases and around 44,000 HEV-related deaths[2]
. Large outbreaks of HEV are often seen in developing countries, but some sporadic cases are now being identified in developed nations[3]
. Chronic infection with HEV most commonly occurs in patients who are either immunosuppressed or immunocompromised.
HEV has at least four different genotypes: 1, 2, 3, and 4[2]
. Genotypes 1 and 2 occur most commonly in developing countries in Africa and Asia, whereas genotypes 3 and 4 primarily cause infections in developed nations[2]
.
This article will outline the difference between acute and chronic HEV infection, as well as the complications associated with the disease.
Transmission
HEV is predominantly transmitted by faecal contamination of drinking water as a result of poor sanitation[2],[4]. Other routes of transmission include consumption of contaminated food, such as raw or undercooked meat (e.g. pork and shellfish) derived from infected animals[5],[6],[7],[8]
and through transfusion of infected blood products (which is more common in highly endemic areas[9]
).
A 2014 UK study that evaluated 225,000 blood donations identified 79 donations that were positive for HEV genotype 3 RNA, which were used to prepare 129 blood components — of which 62 were transfused[10]
. The results showed 43 recipients received HEV-infected transfusions with 42% of patients developing detectable HEV RNA. Consequently, since April 2017, all blood donations in Ireland and the UK are routinely screened for HEV RNA.
Diagnosis
Signs and symptoms
Acute HEV infection
Infection with HEV generally leads to an acute, self-limiting infection and patients are often asymptomatic. Patients who develop symptoms tend to present with non-specific symptoms that are not clinically distinguishable from other types of acute viral hepatitis, including:
- Jaundice;
- Malaise;
- Anorexia;
- Nausea;
- Vomiting;
- Abdominal pain;
- Hepatomegaly;
- Pruritus;
- Arthralgia.
A small proportion of patients develop acute liver failure, and women who are pregnant at the time of infection are at greatest risk.
Chronic HEV infection
A chronic infection is defined by the persistence of HEV RNA in the serum and stool for greater than six months. Patients who are immunosuppressed (following solid organ or bone marrow transplants), and patients with haematological disorders, HIV or conditions requiring heavy doses of immunosuppressive drugs are most susceptible to developing chronic HEV infection[11]
.
Although HEV infection is mainly associated with features of liver disease, extrahepatic manifestations are increasingly recognised; however, causality remains to be established in some cases (see Table 1).
| Table 1: Extrahepatic manifestations of acute and chronic hepatitis E infection | |
| Organ system | Clinical syndrome |
| Neurological |
|
| Kidney |
|
| Haematological |
|
| Other |
|
| Reproduced and adapted with permission from European Association for the Study of the Liver guidelines [11] | |
Laboratory diagnosis
The incubation period of HEV is around 15–60 days[11]
. Three weeks after infection, HEV RNA can be detected in the blood and stools, which occurs shortly before the onset of symptoms. For patients with acute infection, HEV RNA can be detected in the blood for 3–6 weeks, and shedding in the stool can occur for 4–6 weeks.
Definitive diagnosis of HEV infection is usually based on the detection of specific immunoglobulin (Ig) M antibodies to the virus in the patient’s blood[2]
. IgM antibodies, which are short-lived and detected over 4–6 months, appear first. They are followed by the appearance of IgG antibodies, which are long-lasting and have increasing antibody avidity over time.
It is important to test for HEV in any patient presenting with biochemical evidence of hepatitis, irrespective of travel history, because patients with HEV in developed countries can have a locally acquired infection.
Differential diagnosis
Patients presenting with elevated aminotransferases (with or without symptoms of hepatitis) have a broad range of differential diagnoses (see Table 2). Drug-induced liver injury (DILI) is an important differential diagnosis of acute HEV[12]
. In a UK retrospective study of patients with criterion-referenced DILI, 13% of patients with acute HEV were misdiagnosed as having DILI[13]
, highlighting the importance of testing for HEV in patients with this diagnosis.
| Table 2: Differential diagnosis of hepatitis E virus | |
| Infection status | Differential diagnosis |
| Acute infection |
|
| Chronic infection in the immunosuppressed |
|
| Reproduced and adapted with permission from European Association for the Study of the Liver guidelines[11] | |
Management
The management of HEV depends on the immune status of the patient, and whether it is an acute or chronic infection.
Acute HEV infection
There is no specific treatment for acute HEV infection and, in most cases, the virus clears spontaneously. In the rare occurrence of acute liver failure, admission to intensive care or liver transplantation may be required. Early antiviral therapy with ribavirin may shorten the course of the disease and reduce the overall morbidity, but there are limited data to support this in the acute setting[14]
.
Pregnant women with HEV are at an increased risk of acute liver failure, foetal loss and mortality[2]
. These patients should be referred immediately for specialist care.
Chronic HEV infection
This generally only occurs in patients who are immunosuppressed or immunocompromised, and is usually owing to infection with genotype 3 HEV. The aim of treatment is to achieve HEV RNA eradication in both serum and stool (i.e. sustained virologic response [SVR]) 12 weeks after cessation of treatment. Although patients who achieve SVR are cured, they remain at risk of reinfection.
Dose reduction should be the initial step for patients taking immunosuppressants. In a study by Kamar N et al., solid organ transplant recipients with chronic HEV infection, in whom the virus spontaneously cleared, had lower tacrolimus levels and a lower daily steroid dose compared with patients who remained viraemic[15]
. This study led to the concept of reducing immunosuppressive therapy in patients with chronic HEV infection, particularly patients taking therapeutic agents targeting T-cells[15],[16]
.
There are several case reports and case series supporting the use of ribavirin for the treatment of HEV in solid organ transplant recipients[17],[18],[19],[20]
, although there are side effects and contraindications that should be considered (see Box). In a multicentre, retrospective study, 59 solid organ transplant recipients with HEV infection received ribavirin for a median duration of 3 months (range: 1–18 months) at a median dose of 600mg/day (range: 29mg–1200mg/day). SVR was achieved by 78% of patients[20]
. Patients who relapsed after treatment were re-treated for a longer period of 6 months, with one patient receiving 12 months of treatment, and achieved SVR. Doses of ribavirin were adjusted according to kidney function. Although the optimal dose and duration of ribavirin are unknown, a three-month course is an appropriate duration unless patients are heavily immunosuppressed or remain viraemic after one month of treatment[20]
(see Figure 1).
There are some data to suggest that patients with non-transplant immunosuppression (e.g. patients with underlying haematological disorders) with chronic HEV infection can also be successfully treated with ribavirin or pegylated-interferon-alpha, or a combination of both[21],[22],[23],[24],[25]
.
Box: Ribavirin’s side effects and contraindications
Side effects:
- Dose-dependant anaemia — may require blood transfusion or use of erythropoietin;
- Skin reactions;
- Depression;
- Insomnia;
- Dry cough;
- Myalgia;
- Arthralgia.
Contraindications:
- Pregnancy*;
- Breastfeeding;
- A history of severe pre-existing cardiac disease;
- Haemoglobinopathies (e.g. thalassaemia, sickle-cell anaemia).
*Ribavirin is teratogenic and must not be used in pregnant women. For women of childbearing age, ensure appropriate contraception advice is given and that care is taken not to get pregnant during and for six months after the end of treatment. Either male patients or their female partners of childbearing age must, therefore, be counselled to use a form of effective contraception during treatment with ribavirin and after treatment has been stopped.
Source: Electronic Medicines Compendium. Ribavirin 200mg tablets — summary of product characteristics. 2017
[26]
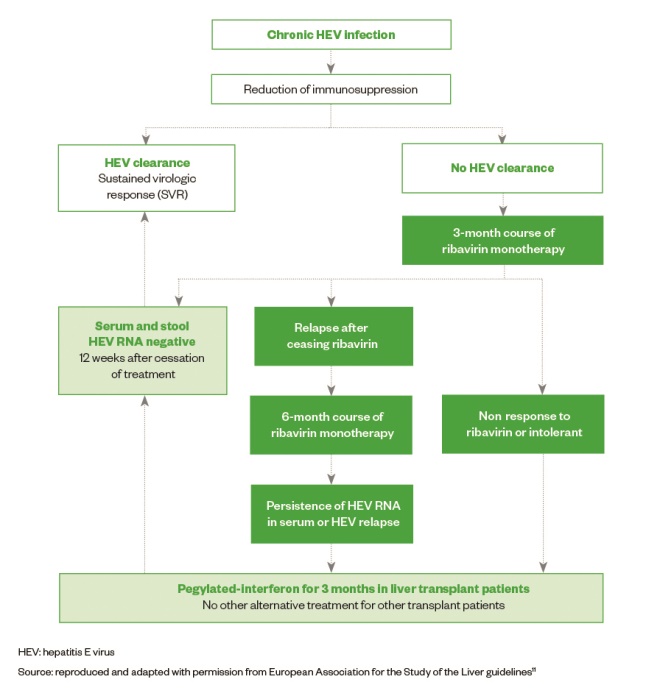
Figure: Treatment of algorithm for chronic hepatitis E virus (HEV) infection in solid organ transplant recipients
Pegylated-interferon-alpha
A three-month course of pegylated-interferon-alpha 2a and 2b has been successfully used to treat a small number of liver transplant recipients who failed treatment with ribavirin, as well as one haemodialysis patient[27],[28],[29]
. However, because of concerns about the immunostimulatory effect, it is generally contraindicated in patients with kidney, pancreas, heart or lung transplantations, owing to the risks of precipitating acute rejection[11]
.
Sofosbuvir
Although developed as treatment for hepatitis C, it has been reported that sofosbuvir has some activity against HEV RNA replication in vitro and has an additive antiviral effect with ribavirin[30]
. However, when a hepatitis C/HEV co-infected patient received a 12-week course of sofosbuvir and daclatasvir, they did not achieve virologic clearance of HEV[31]
. Therefore, it remains unknown if the observations made in vitro will translate into clinical efficacy in vivo.
Prevention
When travelling to regions with poor hygiene and sanitation, and where HEV is endemic, basic recommendations to prevent traveller’s diarrhoea should be adhered to. Pharmacists and healthcare professionals can advise patients that this includes avoiding:
- Water where the purity is unknown;
- Salads;
- Uncooked fruit;
- Unpasteurised dairy products;
- Raw or undercooked meat and seafood.
Furthermore, strict hygiene measures should be followed, such as washing hands thoroughly.
The risk of HEV patient-to-patient transmission is unknown. Sexual transmission in men who have sex with men has been described in two studies[32],[33]
, but in a study with HIV co-infected patients, there was no evidence of sexual transmission[34]
.
A recombinant vaccine against HEV was licensed in China in 2011 and has shown to prevent 97% of episodes of symptomatic acute HEV[35]
. Long-term follow-up showed that the vaccine induced antibodies against HEV and provided protection against HEV for up to 4.5 years[36]
. The vaccine is safe for use in pregnancy[37]
but data are still needed for efficacy in immunocompromised individuals and those with chronic liver disease. There is currently no vaccine licensed for use in the UK.
References
[1] NHS Choices. Hepatitis. 2016. Available at: https://www.nhs.uk/conditions/hepatitis/ (accessed October 2018)
[2] World Health Organization. Hepatitis E fact sheet. 2017. Available at: http://www.who.int/news-room/fact-sheets/detail/hepatitis-e (accessed October 2018)
[3] Clemente-Casares P, Ramos-Romero C, Ramirez-Gonzalez E & Mas A. Hepatitis E virus in industrialized countries: the silent threat. Bio Med Res Int 2016:9838041. doi: 10.1155/2016/9838041
[4] Khuroo MS, Khuroo MS & Khuroo NS. Hepatitis E: discovery, global impact, control and cure. World J Gastroenterol 2016;22(31):7030–7045. doi: 10.3748/wjg.v22.i31.7030
[5] Colson P, Borentain P, Queyriaux B et al. Pig liver sausage as a source of hepatitis E virus transmission to humans. J Infect Dis 2010;202(6):825–834. doi: 10.1086/655898
[6] Lewis HC, Wichmann O & Duizer E. Transmission routes and risk factors for autochthonous hepatitis E virus infection in Europe: a systematic review. Epidemiol Infect 2010;138(2):145–166. doi: 10.1017/S0950268809990847
[7] Li TC, Chijiwa K, Sear N et al. Hepatitis E virus transmission from wild boar meat. Emerg Infect Dis 2005;11(12):1958–1960. doi: 10.3201/eid1112.051041
[8] Berto A, Martelli F, Grierson S & Banks M. Hepatitis E virus in pork food chain, United Kingdom, 2009–2010. Emerg Infect Dis 2012;18(8):1358–1360. doi: 10.3201/eid1808.111647
[9] Khuroo MS, Kamili S & Yattoo GN. Hepatitis E virus infection may be transmitted through blood transfusions in an endemic area. J Gastroenterol Hepatol 2004;19(7):778. doi: 10.1111/j.1440-1746.2004.03437.x
[10] Hewitt PE, Ijaz S, Brailsford SR et al. Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet 2014;384(9956):1766–1773. doi: 10.1016/S0140-6736(14)61034-5
[11] Negro F & Wedemeyer H. EASL clinical practice guidelines on hepatitis E virus infection. J Hepatol 2018;68(6):1256–1271. doi: 10.1016/j.jhep.2018.03.005
[12] Davern TJ, Chalasani N, Fontana RJ et al. Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology 2011;141(5):1665–1672.e1-e9. doi: 10.1053/j.gastro.2011.07.051
[13] Dalton HR, Fellows HJ, Stableforth W et al. The role of hepatitis E virus testing in drugâ€induced liver injury. Aliment Pharmacol Ther 2007;26(10):1429–1435. doi: 10.1111/j.1365-2036.2007.03504.x
[14] Peron JM, Dalton H, Izopet J et al. Acute autochthonous hepatitis E in western patients with underlying chronic liver disease: a role for ribavirin? J Hepatol 2001;54(6):1323–1324. doi: 10.1016/j.jhep.2011.01.009
[15] Kamar N, Abravanel F, Selves J et al. Influence of immunosuppressive therapy on the natural history of genotype 3 hepatitis-E virus infection after organ transplantation.Transplantation 2010;89:353–360. doi: 10.1097/TP.0b013e3181c4096c
[16] Kamar N, Garrouste C, Haagsma EB et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology 2011;140(5):1481–1489. doi: 10.1053/j.gastro.2011.02.050
[17] Mallet V, Nicand E, Sultanik P et al. Brief communication: case reports of ribavirin treatment for chronic hepatitis E. Ann Intern Med 2010;153(2):85–89. doi: 10.7326/0003-4819-153-2-201007200-00257
[18] Kamar N, Rostaing L, Abravanel F et al. Ribavirin therapy inhibits viral replication in patients with chronic hepatitis E virus infection. Gastroenterology 2010;139(5):1612–1618. doi: 10.1053/j.gastro.2010.08.002
[19] Pischke S, Hardkte S, Bode U et al. Ribavirin treatment of acute and chronic hepatitis E: a single centre experience. Liver Int 2013;33(5):722–726. doi: 10.1111/liv.12114
[20] Kamar N, Mallet V, Tripon S et al. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med 2014;370:1111–11120. doi: 10.1056/NEJMoa1215246
[21] Alric L, Bonnet D, Laurent G et al. Chronic hepatitis E virus infection: successful virologic response to pegylated interferon-α therapy. Ann Intern Med 2010;153(2):135–136. doi: 10.7326/0003-4819-153-2-201007200-00256
[22] Alric L, Bonnet D, Beynes-Rauzy O et al. Definitive clearance of a chronic hepatitis E virus infection with ribavirin treatment. Am J Gastroenterol 2011;106:1562–1563. doi: 10.1038/ajg.2011.158
[23] Tavitian S, Peron JM, Huguet F et al. Ribavirin for chronic hepatitis prevention among patients with hematologic malignancies. Emerg Infect Dis 2015;21(8):1466–1469. doi: 10.3201/eid2108.150199
[24] Neukam K, Barreiro P, Macias J et al. Chronic hepatitis E in HIV patients: rapid progression to cirrhosis and response to oral ribavirin. Clin Infect Dis 2013;57(3):465–468. doi: 10.1093/cid/cit224
[25] Hajji H, Gerolami R, Solas C et al. Chronic hepatitis E resolution in a human immunodeficiency virus (HIV)-infected patient treated with ribavirin. Int J Antimicrob Agents 2013;41(6):595–597. doi: 10.1016/j.ijantimicag.2013.02.005
[26] Electronic Medicines Compendium. Ribavirin 200mg tablets — summary of product characteristics. 2017. Available at: https://www.medicines.org.uk/emc/product/7108/smpc (accessed October 2018)
[27] Kamar N, Rostaing L, Abravanel F et al. Pegylated interferon-α for treating chronic hepatitis E virus infection after liver transplantation. Clin Infect Dis 2010;50(5):e30–e33. doi: 10.1086/650488
[28] Haagsma EB, Riezebos-Brilman A, van den Berg AP et al. Treatment of chronic hepatitis E in liver transplant recipients with pegylated interferon alpha-2b. Liver Transpl 2010;16(4):474–477. doi: 10.1002/lt.22014
[29] Kamar N, Abravanel F, Garrouste C et al. Three-month pegylated interferon-alpha-2a therapy for chronic hepatitis E virus infection in a haemodialysis patient. Nephrol Dial Transplant 2010;25(8):2792–279. doi: 10.1093/ndt/gfq282
[30] Dao Thi VL, Debing Y, Wu X et al. Sofosbuvir inhibits hepatitis E virus replication in vitro and results in an additive effect when combined with ribavirin. Gastroenterology 2016;150(1):82–85. doi: 10.1053/j.gastro.2015.09.011
[31] Donnelly MC, Imlach SN, Abravanel F et al. Sofosbuvir and daclatasvir anti-viral therapy fails to clear HEV viremia and restore reactive T cells in a HEV/HCV co-infected liver transplant recipient. Gastroenterology 2017;152(1):300–301. doi: 10.1053/j.gastro.2016.05.060
[32] Montella F, Rezza G, Di Sora F et al. Association between hepatitis E virus and HIV infection in homosexual men. Lancet 1994;344(8934):1433. PMID: 7968090
[33] Payne BA, Medhi M, Ijaz S et al. Hepatitis E virus seroprevalence among men who have sex with men, United Kingdom. Emerg Infect Dis 2013;19(2):333–335. doi: 10.3201/eid1902.121174
[34] Keane F, Gompels M, Bendall R et al. Hepatitis E virus coinfection in patients with HIV infection. HIV Med 2012;13(1):83–88. doi: 10.1111/j.1468-1293.2011.00942.x
[35] Zhu FC, Zhang J, Zhang XF et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 2010;376(9744w):895–902. doi: 10.1016/S0140-6736(10)61030-6
[36] Zhang J, Zhang XF, Huang SJ et al. Long-term efficacy of a hepatitis E vaccine. N Engl J Med 2015;372:914–922. doi: 10.1056/NEJMoa1406011
[37] Wu T, Zhu FC, Huang SJ et al. Safety of the hepatitis E vaccine for pregnant women: a preliminary analysis. Hepatology 2012;55:2038. doi: 10.1002/hep.25522