Jon Findlay, Harm Reduction Lead, Waythrough
After reading this article, you should be able to:
- Understand the importance of naloxone and harm reduction principles in reducing drug-related deaths;
- Understand how naloxone can be distributed to patients at risk of opioid overdose in an acute healthcare setting;
- Address some of the myths associated with naloxone use in people who use drugs.
Introduction
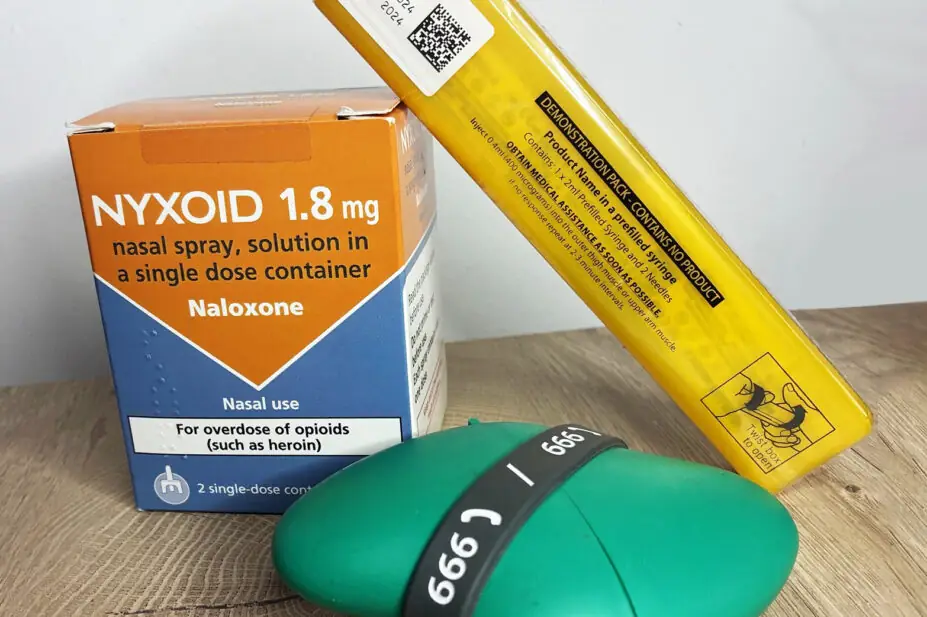
Naloxone is a semisynthetic morphine derivative, which has been used as an emergency antidote for over 40 years. It is a safe and effective specific opioid antagonist that acts competitively at opioid receptors and reverses an opioid overdose, saving an individual’s life1. Naloxone’s distribution was supported as early as 2012 by the Advisory Council on the Misuse of Drugs (ACMD)2 and the World Health Organization (WHO)3, where it appears on the list of essential medicines that countries should fund and supply. In the UK, naloxone is available as an injection or nasal spray (see Box 1)4.
Box 1: Naloxone products available in the UK commonly used by non-healthcare professionals (ampoules have not been included)
Available naloxone formulation include:
- Prenoxad 1mg/ml solution for injection in pre-filled syringe (2ml);
- Nyxoid 1.8mg nasal spray, solution in a single dose container;
- Naloxone 1.36mg nasal spray, solution in single-dose container.
Figure 1: Available naloxone preparations
Distribution through drug services has been prevalent since 2015, following a change in the legislation to allow the supply without prescription to people at risk of opioid overdose or those who may witness one without a prescription1. Community pharmacies delivering substance use services have also distributed naloxone with some schemes emerging as a consequence of the COVID-19 pandemic5.
However, distribution through hospitals, either on a prescription at discharge or under the amended legislation, has been limited. Nevertheless, we know that many people who use drugs (PWUDs) use hospitals for both planned and unplanned admissions. This is a missed opportunity, particularly for PWUDs who may not be engaged with structured drug treatment.
The ACMD review of UK naloxone implementation, published in 2022 and updated in 2023, recognised the limited availability through acute trusts and recommended: “Acute trusts (including emergency departments), mental health trusts and ambulance services should issue take-home naloxone and associated training to those at risk of opioid overdose. Relevant National Institute for Health and Care Excellence guidance should be updated to include appropriate recommendations on naloxone provision.”4
Drug-related deaths are at historic high levels in England and Wales6. Naloxone is a safe and effective medicine that can reduce these deaths. The recommendations from the ACMD and the fact that individuals who misuse substances may not engage with traditional drug services, means that distribution of take-home naloxone through hospitals is an appropriate intervention that should be considered.
This article will outline how distribution of naloxone through hospitals can be implemented, which is demonstrated by the experiences of pharmacy teams in Plymouth and wider Devon. The importance for hospital distribution, harm reduction and some common misconceptions concerning naloxone and its distribution will also be explored.
Framework for introducing naloxone through hospitals
Within University Hospitals Plymouth (UHP) NHS Trust, a substance use steering group (SUSG) was established in 2022. The group, working to a terms of refence framework, has an overarching aim of being “…responsible for ensuring that best evidence-based practice for the management of patients with substance use disorders (including alcohol use disorders) is used and utilised by a well-trained and empathetic practitioner base within UHP NHS Trust to support patient care”.
This group provided the framework for discussion and implementation of its take-home naloxone strategy, which can be used to guide other trusts who are interested in implementing their own naloxone distribution service.
Step 1: Formulary application
Naloxone will need to be included in the formulary as a take-home formulation for use in the community.
At UHP, although naloxone was available on the hospital formulary for emergency use, the availability of naloxone as a take-home formulation for use in the community was not. With support from the SUSG and the pharmacy department, a formulary application was developed for both Prenoxad injection 2mg/2ml and Nyxoid nasal spray 1.8mg. This outlined its place in therapy, inclusion criteria for supply of naloxone (see Box 2), clinical evidence to support the application and a financial evaluation. The application was presented by the lead hepatology pharmacist to the Drug and Therapeutics Committee (DTC) and approved, which became the foundation for introducing it within the hospital.
Box 2: Inclusion criteria for take-home naloxone in the University Hospitals Plymouth (UHP) NHS Trust
The inclusion criteria include:
- Patients admitted to the UHP NHS Trust with an opioid overdose or suspected opioid overdose;
- People who inject drugs and patients who use drugs who may be at risk of an opioid overdose in the community.
Step 2: Developing the standard operating procedure (SOP)
A robust procedural framework needs to be in place to support a wider roll-out.
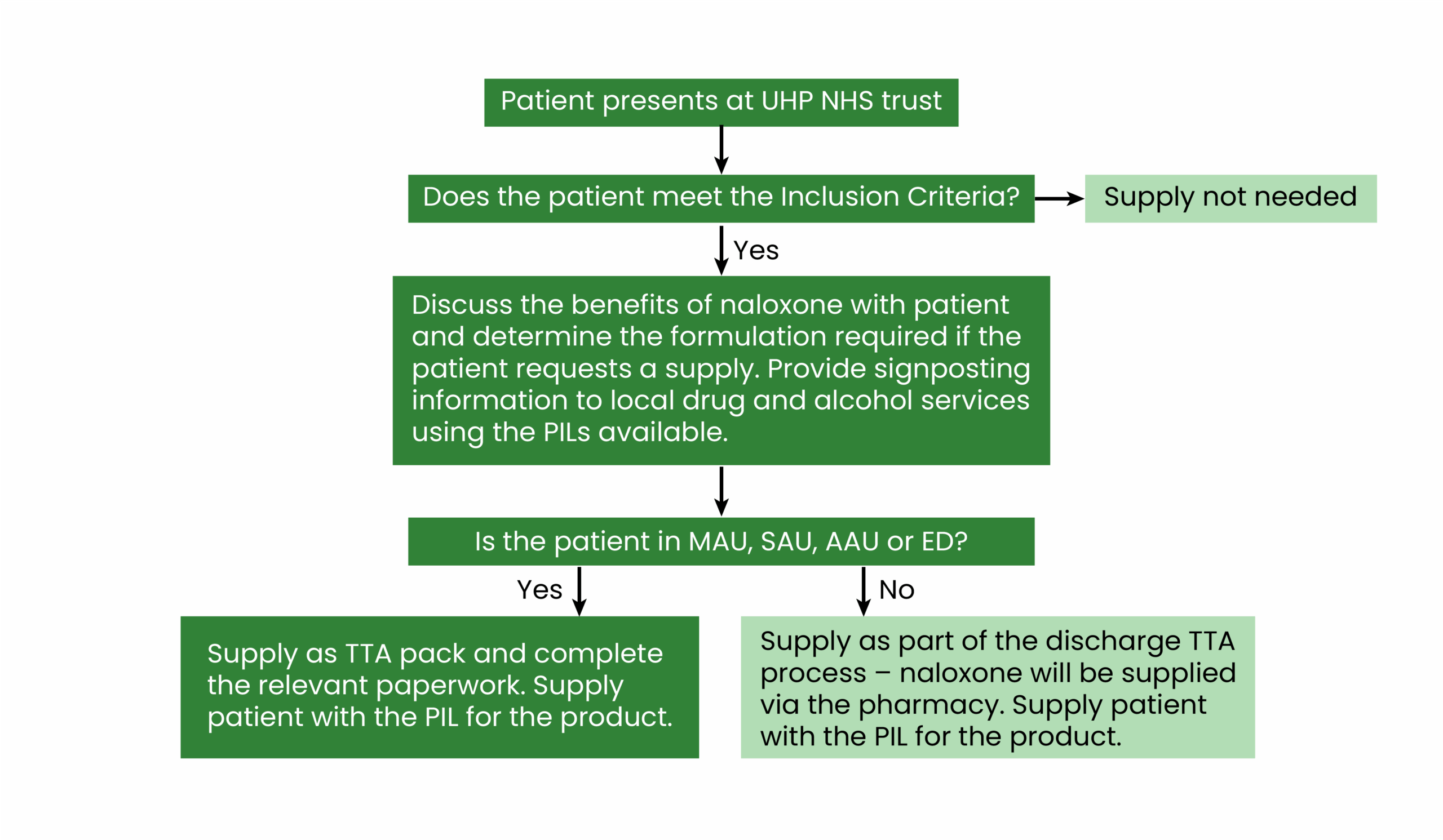
The procedure for the distribution of naloxone was discussed within the SUSG. The SUSG included stakeholders from the wider drug and alcohol service providers, which supported a multidisciplinary and team approach to its distribution. Within the SOP, the following were outlined (see Figure 2):
- Responsibilities and roles: This included the responsibilities of the ward team to identify people during admission who may benefit from a supply of naloxone and for the clinical pharmacist to support the process. Prescribers also held the responsibility to prescribe the naloxone;
- Process at admission and discharge: The SOP outlines how the team should engage the patient on admission and discharge, as well as discuss the benefits of naloxone and provide some basic harm reduction advice;
- Supply process: Supply is made through a discharge summary either through the pharmacy or via pre-labelled to-take-away (TTA) packs (i.e. naloxone packs labelled and ready to be supplied from ward stock when patients want to leave and will not wait for dispensary stock owing to the time constraints);
- Inclusion criteria: This included any patient admitted to the trust with an opioid overdose or suspected opioid overdose or any patient who may be at risk of an opioid overdose in the community;
- Training and support framework (see Figure 2).
Figure 2: A simple flow chart outlining the process of naloxone supply at University Hospitals Plymouth NHS Trust
The purpose and scope with rationale and evidence were also provided within the narrative for the wider audience using the SOP. In addition, best practice for distributing naloxone was identified as part of the SOP. Naloxone was considered for all patients admitted to UHP NHS Trust with an opioid overdose or suspected opioid overdose and for PWUDs who may be at risk of opioid overdose in the community. The decision to supply either the nasal spray or intramuscular injection (IM) was always made in conjunction with the patient, which included a check to understand whether the patient has used a particular formulation before. Familiarity with one formulation over another is often the main driver on which to use.
If the patient had not used naloxone before, a discussion on the two formulations is encouraged; however, it is recognised that people who do not inject, patients who may have trouble assembling the IM injection unit and those concerned about carrying an injection may be more suited to the nasal spray.
A large cohort of patients who are supplied naloxone are familiar with its use, so information and advice does not need to be comprehensive. However, to support the distribution, patient information leaflets were made available for staff supplying naloxone kits and training provided on the use of the different formulations.
Training and support for harm reduction brief interventions were provided. Our intention was not to develop a pseudo-drugs recovery worker but to enable the staff to provide targeted harm reduction advice to individuals while supplying the naloxone. Signposting to local drug and alcohol services was offered with supporting information available if required.
Step 3: Naloxone stock
Immediate stock supply should be sourced for all relevant units and hospital departments. Pharmacy supplies were used for standard supply to patients from wards. However, to facilitate a quick-and-effective turnaround for PWUDs at the medical assessment unit, acute assessment unit, surgical assessment unit and the emergency department, pre-labelled TTA packs were sourced and available for immediate stock supply on these wards.
Owing to the availability of multiple prescribers and the drive for simplicity in the healthcare setting, supplies were made against a prescription from a prescriber, but the delay in supply was mitigated in the departments with a supply of TTA packs.
Step 4: Training and support
Training and support must be tailored to the needs of the staff that engage with patients on the ward. It is also important to consider the local context and whether it would be beneficial to partner with local drug and alcohol service providers. The SUSG developed a robust and comprehensive training and support programme to supplement the launch of the programme.
The aim of the SUSG at the UHP NHS Trust was to support the successful introduction with a comprehensive training and support programme. The group worked with Harbour, the local drug and alcohol service provider in Plymouth, Devon, to develop a training video. This included information on:
- Understanding stigma;
- The different formulations of naloxone and how to use them;
- Harm reduction and patient education;
- Local perspectives, which were delivered by Harbour;
- Challenges and myths (see Box 3);
- Experiences of using naloxone;
- Process for hospital supply;
- Questions.
The e-module naloxone training was also encouraged to be accessed for staff as part of their comprehensive understanding of naloxone, which is available here.
The training video was offered on the UHP NHS Trust website and members of the SUSG visited specific departments to discuss the programme with ward trainers who then cascaded the training to members of the ward team. In addition to this programme, Harbour also offered face-to-face sessions with wards and wider hospital staff for a more comprehensive session on naloxone supply and harm reduction.
Box 3: Myth busting naloxone distribution
- Naloxone increases the risk that people will use more opioids — FALSE: US evidence does not support the claim that naloxone provision could increase or lead to riskier behaviour, while some studies have found decreased use. There is also a considerable body of evidence, mostly from the UK and Australia, to suggest people would not use more heroin if naloxone was available;
- It needs to be injected into the heart like they do in Pulp Fiction — FALSE: The intramuscular injection is administered in the thigh (even through clothes) and a nasal spray is available if the patient does not want to hold an injection;
- The police will always attend an incident when naloxone is used — FALSE: People applying naloxone are always advised to call an ambulance, but the attendance of police is not linked to this2.
Step 5: Launch
The launch involved publication of the SOP and resources, as well as the supply of TTA packs to wards (when appropriate), with visits to the wards to support the distribution of naloxone to PWUDs who accessed the UHP NHS Trust.
Learnings at UHP following launch
TTA naloxone packs were initiated across the UHP NHS Trust in March 2023, thanks to the hard work of the SUSG members in collaboration with primary care drug and alcohol liaison services, which includes ward or service line visits, education session, local trust communications and spreading awareness of the new, enhanced UHP OST policy in-line with iHOST7. Table 1 outlines the numbers distributed and supplied from March 2023 to February 2025.
Figure 3: To-take-away naloxone supplied from the University Hospitals Plymouth NHS Trust to patients between March 2023 to February 2025
Initially in 2023, the uptake and supply of TTA packs was poor. The SUSG team reviewed the potential barriers to this and reimplemented a strategy in October 2023, along with the aid of the UHP pharmacy team to re-engage the UHP NHS Trust. This included strategic locations for access, where patients who may be using illicit substances or on opioid substitution therapy are more likely to reside as part of the secondary care patient journey. These areas included the emergency department, acute medical units, same day emergency care, hepatology, respiratory, urgent treatment centres and minor injury units. Clinical pharmacists on ward areas offered support by adding TTA packs on to TTA discharges, covering all areas where packs are not stocked. This has improved the supply of packs from October 2023, through 2024, and continues into 2025. Future plans include reviewing other areas to stock TTA naloxone packs, including obstetrics, outpatient areas and surgery.
With the continued sustained success of naloxone TTA pack initiation within UHP and the real-world emerging issues surrounding synthetic opiates in the illicit substance arena, it has caused NHS trusts nationally to look into the accessibility of providing naloxone as a TTA pack. UHP being one of the first trusts nationally to have this implemented — and far ahead in the process — has opened interest, communications, networking and shared learning across many platforms with other pharmacist professionals, which include the UK Clinical Pharmacist Association (UKCPA) or direct contact.
Since the implementation, there has been multi-agency collaborative in Devon working towards the synthetic opiate crisis. This has allowed the availability of naloxone TTA packs to expand into primary care drug and alcohol liaison services. More importantly, the use of naloxone TTA packs has expanded into non-healthcare agencies, such as the Devon and Cornwall police force, with front-line officers carrying packs in their vehicles and being included in officer training. Work is continuing to improve awareness of synthetic opiates, education, training, action planning, response to treatment times and community access to naloxone across Devon, supporting patient safety and minimising mortality with emerging drug threats.
Conclusion
Naloxone is a vital tool to reduce drug-related deaths in a vulnerable community when delivered with practical harm reduction advice. Its use is supported by a plethora of national and international bodies. Naloxone is safe and can be effectively distributed outside the traditional drugs and alcohol service hubs, including through hospitals and other acute trust settings.
This article provides pharmacy professionals with a model approach for its introduction in hospitals and acute settings starting at one base and expanding across a county linked to local synthetic opioid drug-related deaths and overdoses. The challenge is to move this forward from theory to practice on a wider field across the UK.
- 1.The Human Medicines (Amendment) (No. 3) Regulations 2015. UK Statutory Instrument 2015 No. 1503. HM Government . 2015. https://www.legislation.gov.uk/uksi/2015/1503
- 2.Consideration of naloxone. Advisory Council on the Misuse of Drugs. 2012. https://assets.publishing.service.gov.uk/media/5a7b2b12ed915d3ed90629e6/consideration-of-naloxone.pdf
- 3.Opioid overdose. World Health Organization. 2023. https://www.who.int/news-room/fact-sheets/detail/opioid-overdose/
- 4.ACMD review of the UK naloxone implementation. Advisory Council on the Misuse of Drugs. 2023. https://www.gov.uk/government/publications/acmd-naloxone-review
- 5.Community pharmacy pilot provides hundreds of take-home naloxone kits in one year. Pharmaceutical Journal. Published online 2021. doi:10.1211/pj.2021.1.104259
- 6.Deaths related to drug poisoning in England and Wales: 2023 registrations. Office of National Statistics. 2024. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsrelatedtodrugpoisoninginenglandandwales/2023registrations
- 7.Improving Hospital Opioid Substitution Therapy (iHOST). London School of Hygiene and Tropical Medicine. 2024. https://www.lshtm.ac.uk/research/centres-projects-groups/ihost
2 comments
You must be logged in to post a comment.

Every hospital needs to do this.
Agree - inspired though iHOST work Jenny and thanks for your continued support.