STEVE GSCHMEISSNER/SCIENCE PHOTO LIBRARY
Prior reading
Before reading this article, you should have read ‘Implementing chimeric antigen receptor T-cell therapy in practice‘.
Learning objectives
After reading this article, you should be able to:
- Describe the stages of the CAR-T therapy pathway from collection of starting material to administration of the final product;
- Understand how CAR-T therapy is manufactured;
- Know the relevant medication restrictions and at which stages of the cycle that drug wash out periods are important;
- Understand which drug factors may influence quality of CAR-T starting material or product.
Cancer learning ‘hub’
Pharmacists are playing an increasingly important role in supporting patients with cancer, working within multidisciplinary teams and improving outcomes.
However, in a rapidly evolving field with numbers of new cancer medicines is increasing and the potential for adverse effects, it is now more important than ever for pharmacists to have a solid understanding of the principles of cancer biology, its diagnosis and approaches to treatment and prevention.
This new collection of cancer content, brought to you in partnership with BeOne Medicines, provides access to educational resources that support professional development for improved patient
Chimeric antigen receptor T (CAR-T) cells are cellular gene therapies utilised for specific relapsed or refractory haematological malignancies. Put simply, the patient’s immune system is redirected via genetic modification of their own T-lymphocytes to recognise and kill malignant cells[1,2].
First introduced into NHS practice in 2018, it remains a rapidly evolving field, with the likelihood of an expansion of clinical indications in coming years[1,2]. As a high cost and high-risk procedure, CAR-T therapy is only delivered in certain hospitals in the UK.
Three therapies are currently approved for NHS use: tisagenlecleucel (Kymriah; Novartis), axicabtagene ciloleucel (Yescarta; Gilead Sciences) and autologous anti-CD19-transduced CD3+ cells (Tecartus; Gilead Sciences)[3–6]. All three target the B-cell surface antigen CD19; therefore, they are only indicated in CD19 positive disease. Treatment indications are relapsed/refractory B-cell acute lymphoblastic leukaemia, diffuse and primary mediastinal large B-cell lymphomas and mantle cell lymphoma[3–6].
Current CAR-T products are manufactured via collection (apheresis) of the patient’s own T lymphocytes through a process known as ‘autologous’ collection. The cells are genetically modified to recognise cell surface antigens on malignant cells, via a chimeric antigen receptor. This results in apoptosis (cell death) of the malignant cell (see Figure 1)[7].
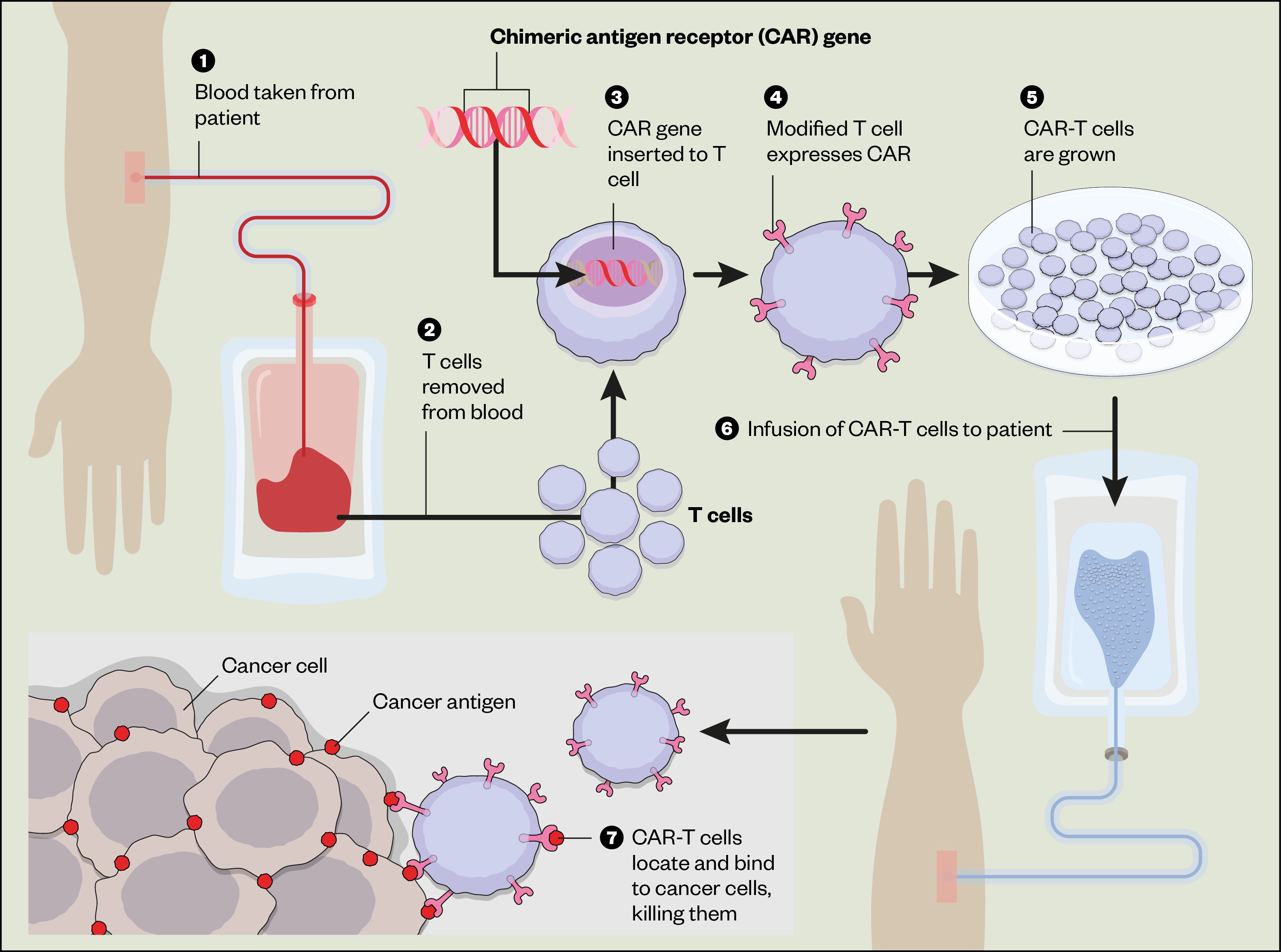
As CAR-T products are licensed medicines, pharmacists are responsible for ensuring that they are of adequate quality for their intended use[8]. In a significant departure from manufacturing methods of traditional medicines, the autologous starting material is provided to the manufacturer by the CAR-T treatment site[9]. There are numerous restrictions regarding permitted medicines and wash-out periods throughout each stage of the CAR-T pathway (see Figure 2). These medication restrictions are the focus of this article.
Box: Counselling on CAR-T therapy, including adverse effects
Pharmacists can provide further counselling around CAR-T therapy and ensure patients are aware of the main adverse effects of CAR -T therapy:
- Cytokine release syndrome (CRS);
- Neurotoxicity.
These predominantly present with mild-to-moderate symptoms, but may become life-threatening, requiring intensive care[10–12]. Rarely, neurotoxicity may be delayed, occurring following discharge from hospital[13].
Adverse effects also include risk of serious infection and bleeding owing to immunosuppression and thrombocytopenia respectively[10–12]. Patients should be made aware of longer-term implications, such as avoiding driving for eight weeks following treatment, life-long avoidance of organ/blood donation and to carry their CAR-T patient alert card. Implementation of CAR-T therapy in practice is further outlined in ‘Implementing chimeric antigen receptor T-cell therapy in practice’.
CAR-T cell medication restrictions
CAR-T cells are living drugs, manufactured from T lymphocytes, meaning that they are extremely vulnerable to the effects of other medicines, which may impair or destroy lymphocytes[14].
It is therefore important to consider the effect of concomitant medicines on lymphocyte quality and quantity, starting from the period prior to the apheresis procedure, until post-CAR-T administration, to maximise CAR-T expansion and persistence in vivo. To assist with these considerations, the following guidance should be followed, outlining the restrictions for concomitant medicines.
Sources of information
Information on medication restrictions is provided by CAR-T manufacturers and best practice recommendations from the European Society for Blood and Marrow Transplantation, the European Haematology Association and American Society for Transplantation and Cellular Therapy[14–16]. Recommendations are based on published clinical trial data, clinical trial protocols, observational data or best practice. Evidence continues to evolve, and guidance may change rapidly and differ between products/indication. The patient’s clinical condition and disease status must be considered[9,14]. To provide a single reference point for medication restrictions, the Pan UK Pharmacy Working Group for ATMPs and Midland-Wales Advanced Therapy Treatment Centre have produced a summary document for licensed CAR-T products, accessible via the Specialist Pharmacy Service (SPS)[17]. This and/or manufacturer’s guidance should be consulted for clinical queries, as this article provides an overview only.
General considerations for concomitant medicines
Before the CAR-T process begins, until the period following its administration, consideration needs to be given to the management of any concomitant drug therapies that the patient is receiving, including:
- The impact of the drug on T-cells and/or on CD19. For example, blinatumumab, an anti-CD19 monoclonal antibody, should be avoided as it will deplete cells with the CD19 cell surface antigen, which is required for CAR-T activation;
- The potential for lymphopenia (reduction in lymphocytes levels). For example, bendamustine may cause prolonged lymphopenia and affect apheresis collection;
- The pharmacokinetics (including half-life) and pharmacodynamics of the drug;
- Whether five half-lives will be a sufficient wash-out period. Drug clearance will be complete within five half-lives but the effect of a drug on T cells may persist beyond this. For example, the effect of alemtuzumab on lymphocytes is likely to extend beyond its elimination half-life;
- The potential to cause organ toxicity or additive adverse effects. For example, high-dose chemotherapy is not advised owing to the risk of organ toxicity, which may preclude the patient from meeting fitness criteria to proceed with CAR-T treatment[9,14–16].
Concomitant medication considerations specific to each stage of the CAR-T process
Pre-apheresis (T-cell collection)
The quality of the patient’s lymphocytes, as the starting material, is integral to the quality and potential efficacy of the final product[18].
Prior chemotherapy may reduce lymphocyte quality and count. Recovery from cytopenias and an adequate lymphocyte count of at least 0.2 x 109/L pre-apheresis is generally advised, but emerging data supports apheresis in patients with lower lymphocyte counts[3,14]. Wash-out periods for chemotherapy of two weeks or five half-lives is recommended by CAR-T manufacturers as a rule, but this is overridden by drug specific guidance[19–21]. For example, lymphotoxic drugs, such as fludarabine, bendamustine, clofarabine and alemtuzumab, are recommended to be avoided pre-apheresis, or require lengthy wash-out periods owing to prolonged effects on T-cells[9,15,16]. In these instances, where treatment is necessary to maintain disease control, alternative therapy should be discussed with the CAR-T treatment centre team.
Corticosteroids should be avoided for at least seven days prior to apheresis owing to the ability to cause lymphocyte apoptosis (cell death)[9,14,19,20].
Immunosuppressants should be stopped at least two weeks prior to apheresis owing to an anti T-cell effect[15].
Pre-emptive apheresis for high-risk patients (e.g. severely immunocompromised individuals) is being explored, aiming to increase the quality of T-cells collected, but has cost, regulatory and infrastructure implications[3,14]. The T-cells would require storage under strict conditions in an accredited facility, meeting regulatory standards to maintain cell viability. This would require significant storage space at a considerable cost if widely adopted. It is also possible some high-risk patients will be unable to eventually proceed to CAR-T treatment, owing to rapidly progressive disease.
Allogeneic CAR-T products, utilising lymphocytes from healthy donors, continue to be evaluated as ‘off-the-shelf’ alternatives to autologous products. As healthy donors are less likely to be receiving treatment for pre-existing medical conditions, wash-out periods pre-apheresis may be shortened and the starting material may be of higher quality[22].
Considerations pre- and post- CAR-T infusion
As a living drug, which is intended to expand and persist for a period in vivo, the CAR-T product is highly sensitive to the effects of medicines administered either prior to, or following, CAR-T infusion[10–12,19,20,23].
Lymphodepletion chemotherapy administered to prepare patients for CAR-T administration is excluded from wash-out recommendations [10, 11, 12]. Further details of lymphodepletion chemotherapy are provided within the relevant CAR-T product summary of product characteristics[10–12].
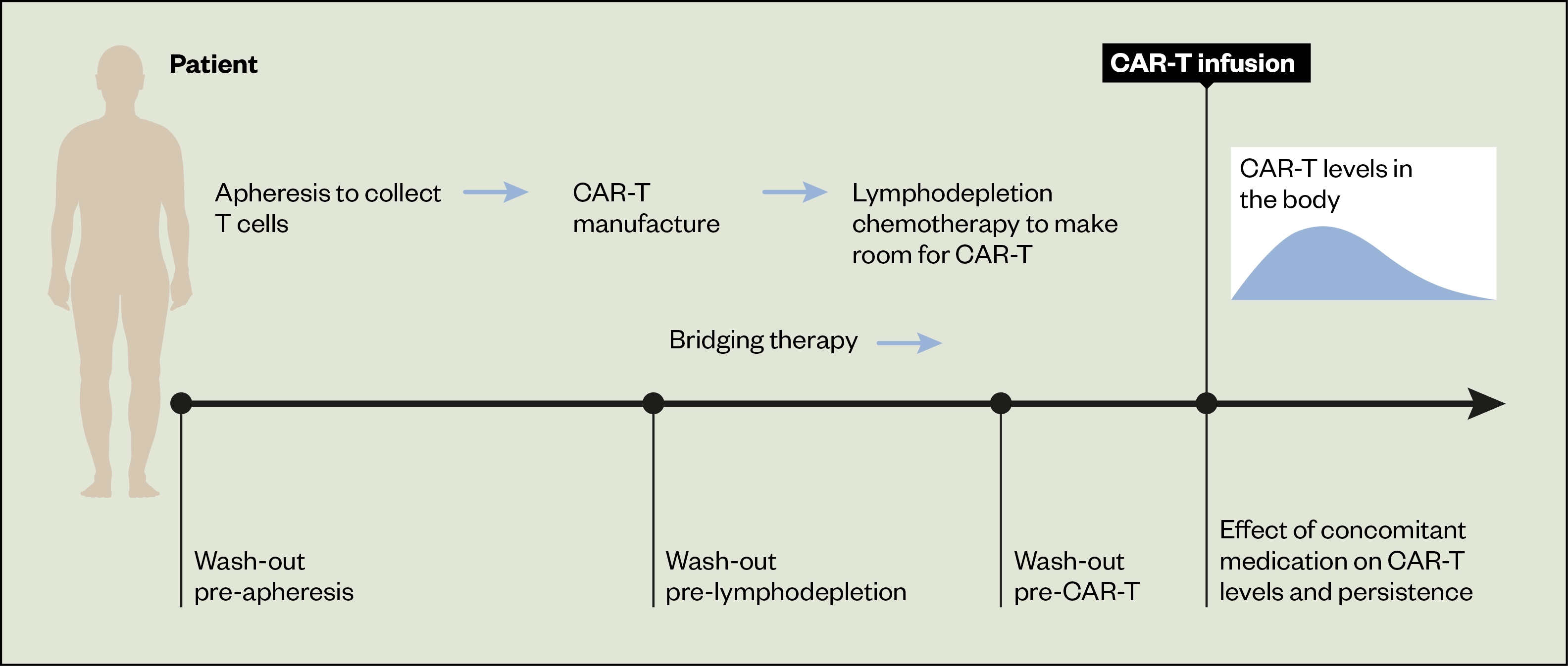
Bridging therapy
Owing to the significant CAR-T manufacturing time (three to four weeks), bridging therapy is administered between apheresis and lymphodepletion/CAR-T administration (see Figure 2). It may be omitted in patients with low volume or indolent disease. Bridging therapy consists of radiotherapy, corticosteroids, systemic anti-cancer treatments or a combination of these treatments, with the intent to provide disease control rather than elucidating a remission[16]. Highly toxic regimens should be avoided as adequate organ function and absence of serious infection is required to proceed to CAR-T treatment[15]. Bridging therapy was not permitted in the pivotal ZUMA-1 study of axicabtagene ciloleucel in lymphoma and so recommendations may be informed by ongoing clinical trial protocols or best practice guidelines[24].
Blinatumumab is not recommended as bridging therapy owing to the potential for CD19 antigen escape[16,23]. Presence of the CD19 B-cell surface antigen is required for CAR-T engagement with the malignant B-cell and apoptosis.
Wash-out periods for bridging chemotherapy are often drug and dose specific. High dose chemotherapy should be stopped at least three to four weeks prior to lymphodepletion to reduce the risk of additional toxicity and prolonged cytopenias[14]. Two week ‘wash-outs’ are recommended for rituximab, bendamustine and polatuzumab prior to lymphodepletion for axicabtagene ciloleucel and five days for ibrutinib prior to lymphodepletion for autologous anti-CD19-transduced CD3+ cells[19,20].
Corticosteroids
Where corticosteroid bridging is utilised, wash-outs of three to ten days are advised prior to CAR-T infusion[19,20,23].
Corticosteroids should not be prescribed as anti-emetics or for prophylaxis of infusion-related reactions prior to CAR-T administration, but intermittent topical, inhaled or intranasal corticosteroids are permitted[10–12,14].
Corticosteroids are indicated in the treatment of CAR-T-induced toxicity; further information can be found in a previous article here. Although CAR-T manufacturers state the use of corticosteroids does not appear to adversely affect CAR-T expansion or persistence, this remains an area of debate owing to the anti-lymphocyte action of corticosteroids[10–12,25,26].
Recent evidence has demonstrated that earlier intervention with corticosteroids in the treatment of CAR-T toxicity and prophylactic use of corticosteroids to reduce toxicity did not alter the pharmacokinetics or efficacy of axicabtagene ciloleucel and may reduce overall corticosteroid exposure[27,28]. However, these studies included relatively small, non-randomised cohorts and UK recommendations remain unchanged.
Other medicines and alternative therapies
Systemic immunosuppressants may impair CAR-T viability via an anti T-cell action[14]. The glycoprotein granulocyte colony stimulating factor may exacerbate CAR-T toxicity; therefore, it should be avoided for at least two to three weeks following CAR-T administration and only prescribed once any CAR-T toxicity has resolved[11,14]. Alternative therapies should be ceased as far as possible in advance as an effect on apheresis or CAR-T efficacy cannot be excluded[17]. Wash-out recommendations for radiotherapy, intrathecal chemotherapy, checkpoint inhibitors and other medications are available via SPS guidance for CAR-T medication restrictions[17].
Pharmacists should instruct patients to communicate changes in medicines to their pharmacy team, including any delays, ensuring patients are aware that this includes non-prescribed and alternative therapies.
Vaccinations
CAR-T patients remain susceptible to infection post-treatment, owing to B-cell aplasia and cytopenias. Despite a risk of incomplete response to vaccination (including to COVID-19 vaccination) and a lack of clinical data, European guidelines advise general vaccination may still reduce infection rates and improve outcomes in this population[3,14].
Live vaccinations should be avoided from six weeks prior to starting lymphodepletion chemotherapy until immune reconstitution has occurred following CAR-T treatment, owing to the risk of infection in a severely immunocompromised CAR-T recipient[10–12].
Killed/inactivated vaccines may be administered at six months after CAR-T therapy and at least two months after the last immunoglobulin replacement dose, where applicable, to ensure that the patient has a sufficiently functioning immune system to mount a response to the vaccine and that residual circulating antibodies from immunoglobulin administration do not impact the efficacy of the vaccine[14,29].
Advice on COVID-19 vaccination in the CAR-T cell setting has changed rapidly as new information evolves, but current best practice guidelines state:
- Patients should be vaccinated prior to admission for CAR-T therapy, where possible, following the normal COVID-19 vaccine schedule;
- Patients who have received COVID-19 vaccination prior to CAR-T therapy are considered unvaccinated post treatment and should be offered re-vaccination starting at three to six months post CAR-T infusion[30].
CAR-T patients should be advised to be cautious in their contacts despite COVID-19 vaccination, as efficacy in this patient group is yet to be fully defined and many patients lack a fully functioning immune system[30].
Role of pharmacists and pharmacy teams in CAR-T medication restrictions
As medicines experts, pharmacists are essential in ensuring the safety and efficacy of CAR-T therapy. Pharmacists and pharmacy teams can respond to patient questions and educate other healthcare professionals caring for CAR-T patients on medication restrictions (see Table and ‘Best practice‘).
The wider role of pharmacists in CAR-T therapy has previously been covered here.
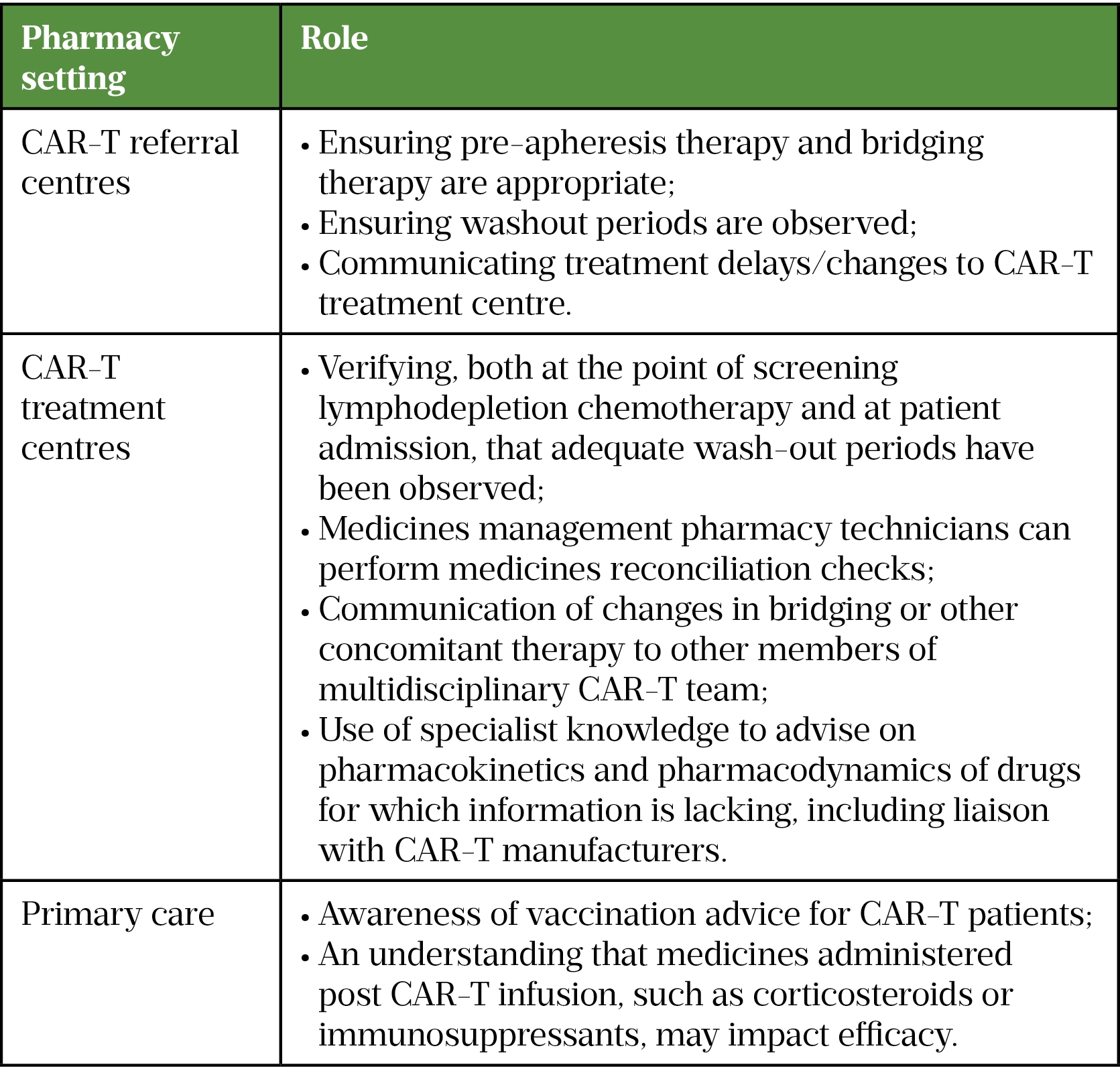
Box: Best practice
- Maintain awareness of medication restrictions and any updates as new CAR-T cell products are launched or licensed indications are extended for existing products;
- Maintain good communication between treatment and referral centres regarding prior and bridging therapy;
- Medicines reconciliation is vital at the point of admission for CAR-T cell therapy, but obtaining a detailed drug history is also an integral part of a patient’s work-up pre-apheresis;
- Pharmacists can educate other healthcare professionals on the importance of adequate wash-out periods for prior therapies.
Acknowledgements
The authors would like to thank the Pan UK Pharmacy Working Group for ATMPs, Midlands-Wales Advanced Therapy Treatment Centre, Specialist Pharmacy Service and University Hospital of Wales Medicines Information teams, University Hospitals Bristol and Weston NHS Foundation Trust, University Hospital of Wales.
Financial and conflicts of interest disclosure
Nia Evans has received an educational grant from KITE/Gilead. The authors have no other relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this manuscript.
- 1Service Specification 170100S (draft interim). Axicabtagene Ciloleucel Chimeric Antigen Receptor T Cell (CAR T) Therapy for the treatment of adult patients with relapsed or refractory large B-cell lymphoma. NHS England and Improvement. 2018.https://www.england.nhs.uk/wp-content/uploads/2018/12/Axicabtagene-Ciloleucel-Chimeric-Antigen-Receptor-T-Cell-CAR-T-Therapy-for-the-treatment-of-adult-patients-wit.pdf (accessed Jul 2022).
- 2Service Specification 170099SS (draft interim). Tisagenlecleucel Chimeric Antigen Receptor T Cell (CAR T) Therapy for ALL and DLBCL. NHS England and Improvement. 2018.https://www.england.nhs.uk/wp-content/uploads/2018/12/Tisagenlecleucel-Chimeric-Antigen-Receptor-T-Cell-CAR-T-Therapy-for-ALL-and-DLBCL.pdf (accessed Jul 2022).
- 3Axicabtagene ciloleucel for treating diffuse large B-cell lymphoma and primary mediastinal large B-cell lymphoma after 2 or more systemic therapies Technology appraisal guidance [TA559]. National Institute for Health and Care Excellence. 2019.https://www.nice.org.uk/guidance/ta559 (accessed Jul 2022).
- 4Tisagenlecleucel for treating relapsed or refractory B-cell acute lymphoblastic leukaemia in people aged up to 25 years Technology appraisal guidance [TA554]. National Institute for Health and Care Excellence. 2018.https://www.nice.org.uk/guidance/ta554 (accessed Jul 2022).
- 5Tisagenlecleucel for treating relapsed or refractory diffuse large B-cell lymphoma after 2 or more systemic therapies Technology appraisal guidance [TA567]. National Institute for Health and Care Excellence. 2019.https://www.nice.org.uk/guidance/ta567 (accessed Jul 2022).
- 6Autologous anti-CD19-transduced CD3+ cells for treating relapsed or refractory mantle cell lymphoma Technology appraisal guidance [TA677]. National Institute for Health and Care Excellence. 2021.https://www.nice.org.uk/guidance/ta677 (accessed Jul 2022).
- 7CAR T-Cell Therapy. National Cancer Institute. 2018.https://visualsonline.cancer.gov/details.cfm?imageid=12069 (accessed Jul 2022).
- 8Black A. (ATMPs)-The Role of Pharmacy in the Successful Delivery of Advanced Therapy Medicinal Products Information for Chief Pharmacists. Specialist Pharmacy Service. 2017.https://www.sps.nhs.uk/articles/atmps-the-role-of-pharmacy-in-the-successful-delivery-of-advanced-therapy-medicinal-products-atmps-information-for-chief-pharmacists/ (accessed Jul 2022).
- 9CAR-T Kymriah/Tisagenlecleucel/CTL019 and clinical CAR-T cell products. Novartis Technical Operations 2013.
- 10Yescarta. Electronic medicines compendium. 2022.https://www.medicines.org.uk/emc/product/ (accessed Jul 2022).
- 11Kymriah cells dispersion for infusion. Electronic medicines compendium. 2022.https://www.medicines.org.uk/emc/product/9456 (accessed Jul 2022).
- 12Tecartus (Great Britain). Electronic medicines compendium. 2022.https://www.medicines.org.uk/emc/product/11987 (accessed Jul 2022).
- 13Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy — assessment and management of toxicities. Nat Rev Clin Oncol. 2017;15:47–62. doi:10.1038/nrclinonc.2017.148
- 14Hayden PJ, Roddie C, Bader P, et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA). Annals of Oncology. 2022;33:259–75. doi:10.1016/j.annonc.2021.12.003
- 15Jain T, Bar M, Kansagra AJ, et al. Use of Chimeric Antigen Receptor T Cell Therapy in Clinical Practice for Relapsed/Refractory Aggressive B Cell Non-Hodgkin Lymphoma: An Expert Panel Opinion from the American Society for Transplantation and Cellular Therapy. Biology of Blood and Marrow Transplantation. 2019;25:2305–21. doi:10.1016/j.bbmt.2019.08.015
- 16Kansagra AJ, Frey NV, Bar M, et al. Clinical utilization of Chimeric Antigen Receptor T-cells (CAR-T) in B-cell acute lymphoblastic leukemia (ALL)–an expert opinion from the European Society for Blood and Marrow Transplantation (EBMT) and the American Society for Blood and Marrow Transplantation (ASBMT). Bone Marrow Transplant. 2019;54:1868–80. doi:10.1038/s41409-019-0451-2
- 17Black A. Medication Restrictions for Patients Having CAR-T Cell Therapy V3. Specialist Pharmacy Service. 2021.https://www.sps.nhs.uk/articles/medication-restrictions-for-patients-having-car-t-cell-therapy/ (accessed Jul 2022).
- 18Noaks E, Peticone C, Kotsopoulou E, et al. Enriching leukapheresis improves T cell activation and transduction efficiency during CAR T processing. Molecular Therapy – Methods & Clinical Development. 2021;20:675–87. doi:10.1016/j.omtm.2021.02.002
- 19Yescarta (axicabtagene ciloleucel) — Cessation of prior therapies. Gilead Sciences Ltd 2021.
- 20Tecartus (autologous anti-CD19-transduced CD3+ cells, KTE-X190 — Cessation of prior therapies. Gilead Sciences Ltd 2020.
- 21Guidance for Therapy and Drug Washout Prior to Leukapheresis for Tisagenlecleucel Manufacture. Novartis Oncology 2021.
- 22Caldwell KJ, Gottschalk S, Talleur AC. Allogeneic CAR Cell Therapy—More Than a Pipe Dream. Front. Immunol. 2021;11. doi:10.3389/fimmu.2020.618427
- 23Bridging therapy: Recommended washout time prior to tisagenlecleucel infusion. Novartis Pharmaceutical UK Ltd 2021.
- 24Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377:2531–44. doi:10.1056/nejmoa1707447
- 25Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-Care Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma: Results From the US Lymphoma CAR T Consortium. JCO. 2020;38:3119–28. doi:10.1200/jco.19.02104
- 26Strati P, Ahmed S, Furqan F, et al. Prognostic impact of corticosteroids on efficacy of chimeric antigen receptor T-cell therapy in large B-cell lymphoma. Blood. 2021;137:3272–6. doi:10.1182/blood.2020008865
- 27Topp MS, Meerten T, Houot R, et al. Earlier corticosteroid use for adverse event management in patients receiving axicabtagene ciloleucel for large B‐cell lymphoma. Br J Haematol. 2021;195:388–98. doi:10.1111/bjh.17673
- 28Oluwole OO, Bouabdallah K, Muñoz J, et al. Prophylactic corticosteroid use in patients receiving axicabtagene ciloleucel for large B‐cell lymphoma. Br J Haematol. 2021;194:690–700. doi:10.1111/bjh.17527
- 29Hill JA, Seo SK. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood. 2020;136:925–35. doi:10.1182/blood.2019004000
- 30BSBMTCT AND COVID. BRITISH SOCIETY OF BLOOD AND MARROW TRANSPLANTATION AND CELLULAR THERAPY. 2022.https://bsbmtct.org/bsbmtct-and-covid/ (accessed Jul 2022).