Shutterstock.com
After reading this article, you should be able to:
- Understand why neonates are at increased risk of infections;
- Recognise the symptoms of sepsis;
- Know the difference between early- and late-onset sepsis and how the conditions are managed;
- Understand best practice for infection prevention in neonates.
Neonatal infections are a significant cause of morbidity and mortality worldwide, especially in preterm and low-birth-weight infants1. The prevalence and morbidity rates vary depending on factors such as gestational age, birth weight, healthcare settings and regional disparities2.
Long-term complications associated with neonatal sepsis, include:
- Neurodevelopmental impairment: increased risk of cerebral palsy, cognitive delays and hearing/vision impairments3;
- Bronchopulmonary dysplasia (BPD), particularly in preterm infants;
- Increased risk of necrotising enterocolitis (NEC), a life-threatening gastrointestinal condition. More information about NEC can be found in ‘Gastrointestinal complications associated with prematurity‘;
- Prolonged hospital stays, leading to increased healthcare costs and further complications4.
Neonates (especially preterm and low-birthweight infants) are significantly more susceptible to infections owing to several physiological and immunological factors5. Their underdeveloped immune system, limited physical barriers and exposure to pathogens during birth and hospital stays all contribute to this increased vulnerability6.
Reasons for increased susceptibility can be seen in Figure 16.
Sepsis can be classified as either early-onset sepsis (EOS) or late-onset sepsis (LOS). This article will cover the recognition and management of both, as well as the pharmacist’s role in the prevention of these conditions.
Early-onset sepsis
Early-onset sepsis (EOS) occurs within the first 72 hours of life7. It has an incidence of 0.5 to 1.0 per 1,000 live births in high-income countries8. Rates of EOS are higher in very preterm infants (<32 weeks gestation), with rates of 13.5 per 1,000 live births9.
When there is a strong clinical suspicion of sepsis, neonates must receive appropriate treatment promptly to reduce the mortality and morbidity associated with EOS.
If any risk factors (see Box 17) or clinical indicators (see Table 17) for EOS are present, the following steps should be taken:
- A clinical assessment of the infant should be immediately performed;
- The maternal and neonatal history should be reviewed;
- The baby should be physically examined, including an assessment of vital signs7.
This assessment should guide the clinical management of the baby.
Box 1: Risk factors for early-onset neonatal infection
- Suspected or confirmed infection in another baby in the case of a multiple pregnancy is a red flag risk factor;
- Invasive group B streptococcal (group B strep) infection in a previous baby or maternal group B strep colonisation, bacteriuria or infection in the current pregnancy;
- Preterm birth following spontaneous labour before 37 weeks’ gestation;
- Confirmed rupture of membranes for more than 18 hours before a preterm birth;
- Confirmed prelabour rupture of membranes at term for more than 24 hours before the onset of labour;
- Intrapartum fever higher than 38°C, if there is suspected or confirmed bacterial infection;
- Clinical diagnosis of chorioamnionitis.
In babies with any one red-flag risk factors or clinical indicators, or with two or more ‘non-red-flag’ risk factors or clinical indicators, further investigations (e.g. C-reactive protein levels, blood culture, lumbar puncture, eye swab for purulent eye discharge or skin swab for purulent umbilical discharge/periumbilical cellulitis) should be completed before starting antibiotics — antibiotics should be started immediately (i.e. not delayed until test results are available)7.
In babies without red flags and with only one risk factor or one clinical indicator present, clinical judgement should be used to decide whether it is safe to withhold antibiotics and whether the baby’s vital signs and clinical condition need to be monitored. If monitoring is needed, it should be continued for at least 12 hours using a newborn early warning system, such as the Newborn Early Warning Trigger and Track 2 (NEWTT2) framework, which was published by British Association of Perinatal Medicine (BAPM) in 202310.
For more information on determining the need to start antibiotic treatment in babies with suspected EOS, please see the NICE guidance7.
The Kaiser Permanente Sepsis Risk Calculator (KP-SRC) is an alternative assessment tool that uses the population’s background incidence of EOS, objective information at birth and the infant’s clinical presentation to evaluate risk of neonatal EOS in infants >34 weeks gestation11. It has been shown to reduce the use of antibiotics and can be used to determine risk of neonatal sepsis for babies born after 34 weeks being cared for in a neonatal unit, transitional care unit or a postnatal ward. Studies have shown a 40–50% reduction in antibiotic use in some hospitals using the calculator12. Reducing unnecessary antibiotics may help preserve the neonatal microbiome and lower risks of conditions such as NEC and antibiotic resistance.
After calculating the risk, neonates are categorised as:
- No evaluation needed (low risk);
- Clinical monitoring only (moderate risk);
- Empiric antibiotics recommended (high risk)11.
Antibiotic prescribing
The organisms that cause EOS in neonates are predominantly those that colonise the vagina or lower gastrointestinal tract in mothers; Group B strep and Escherichia coli (E coli) are the main organisms of concern13.
Intravenous benzylpenicillin and gentamicin are the drugs of choice for managing EOS7. Benzylpenicillin has excellent activity against gram-positive organisms (such as Group B strep) and gentamicin has good cover against gram-negative organisms (such as E coli). Benzylpenicillin is administered at a dose of 25–50mg/kg at 8- or 12-hourly intervals, depending on age and the severity of the infection14.
To target gram-negative organisms in neonates aged under 7 days, gentamicin is usually administered as a 5mg/kg dose every 36 hours; however, the dose interval can be shortened to 24-hourly if the baby is severely unwell or increased to 48-hourly (or more) if the baby has renal issues and is struggling to eliminate gentamicin15. Therapeutic drug monitoring is recommended because gentamicin has a narrow therapeutic range. Amikacin can be used as an alternative to gentamicin if there is a high incidence of gentamicin resistance locally.
In areas where therapeutic drug monitoring of aminoglycosides may be challenging (e.g. owing to a lack of medical staff or access to laboratory testing), a broad-spectrum third-generation cephalosporin, such as cefotaxime, can be used to treat early-onset sepsis, owing to its broad-spectrum antimicrobial activity, covering most of pathogens implicated in neonatal sepsis16.
If there are any indicators of Listeria infection (e.g. dark-coloured, foul-smelling amniotic fluid), benzylpenicillin can be changed to amoxicillin 50–100mg/kg 8 or 12-hourly (depending on age). Listeria is an uncommon but serious cause of neonatal sepsis and meningitis17.
Complications and mortality
Complications from EOS depend on the causative organism, gestational age, and how quickly treatment is initiated. While many term babies recover well with prompt antibiotic treatment, preterm and critically ill neonates have a higher risk of severe complications.
The overall mortality from EOS is around 2–3% in term neonates and around 15–30% in preterm neonates (<32 weeks gestation)18. Gram-negative sepsis (e.g. E coli) has a higher mortality rate of around 20-30%, especially in preterm neonates19. The mortality rate of Listeria is similar to this, but rare.
Late onset sepsis
Late onset sepsis (LOS) occurs after 72 hours of life and is typically acquired from the hospital (nosocomial) or community20. Incidence in term infants is 1.6% of live births21. It is more common in preterm neonates owing to prolonged hospital stays, invasive procedures and immature immune systems. In preterm neonates, particularly very low birth weight (<1,500g) infants, LOS can occur in 12–50% of cases21. Mortality rates are 10–20%, but can be as high as 35% in extremely low birth weight (<1,000g) neonates21,22.
Gram-positive bacteria (e.g. Coagulase negative staphylococcus (CoNS), Staphylococcus aureus, Enterococcus) are the most common causes of LOS, especially in neonatal intensive care units (NICUs)23,24. Gram-negative bacteria (e.g. E. coli, Klebsiella, Pseudomonas) are more aggressive and associated with higher mortality25. Fungal infections (Candida) can occur in very preterm neonates with prolonged antibiotic use and/or central lines26. Risk factors, such as central lines, ventilation and NEC, influence the causative organisms27.
Signs and symptoms
The clinical signs and symptoms of late-onset sepsis are wide-ranging, as outlined in Table 27.
LOS in neonates can progress rapidly, meaning that early recognition and treatment are critical. Even one red-flag symptom or multiple mild symptoms should prompt urgent evaluation and empirical antibiotic therapy7. If any red-flag symptoms are present, sepsis should be assumed until proven otherwise.
Red-flag symptoms of LOS can be seen in Figure 27.
If any red-flag symptoms are present, the baby should receive a sepsis screen and antibiotics should be started within one hour; waiting for test results should not delay starting treatment7.
Empirical treatment
For babies with suspected late-onset neonatal infection, a combination of narrow-spectrum antibiotics (such as IV flucloxacillin plus gentamicin) are usually given as first-line treatment7. Flucloxacillin can be replaced with vancomycin or teicoplanin when there are additional concerns about gram positive organisms; for example, when infants have indwelling plastic lines, such as central venous catheters at risk of infections with CoNS, which is common in preterm infants28.
If NEC is suspected, an antibiotic that is active against anaerobic bacteria (such as metronidazole) should be added7. If first-line treatments are ineffective, switching to an alternative agent such as meropenem should be considered, as this has good cover against gram-negative bacteria, such as Klebsiella and Pseudomonas29.
Treatment should be guided and adjusted based on blood culture results30. If Candida species are suspected, fluconazole or liposomal amphotericin B should be added31.
Duration of treatment depends on the causative organism and severity of infection, for example:
- Bacteraemia (without meningitis): 7–10 days;
- Meningitis: 14–21 days21;
- Fungal sepsis: 4–6 weeks32.
NICE management recommendations for neonates with suspected late-onset sepsis are outlined in Figure 37.
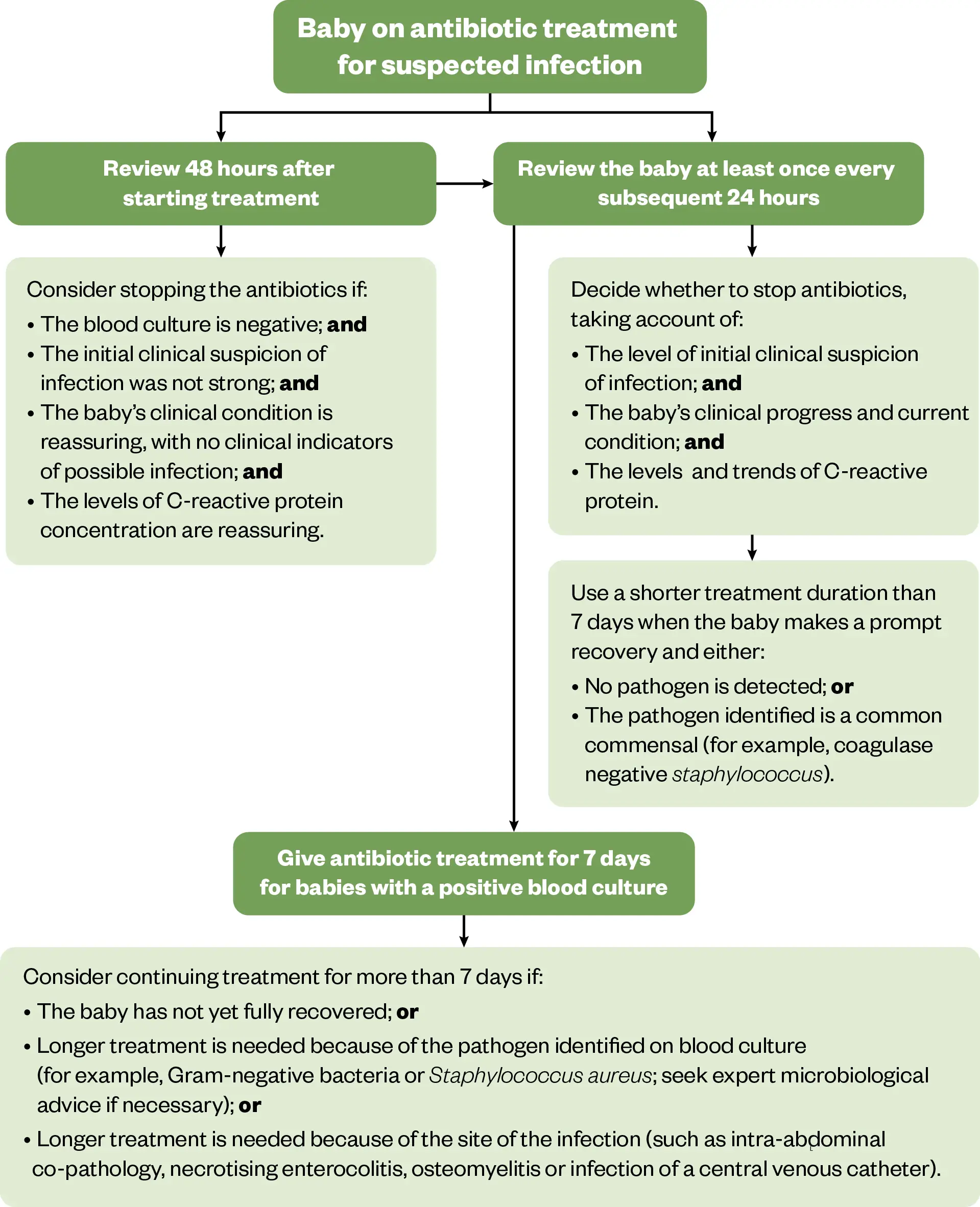
The Pharmaceutical Journal (adapted from NICE)
NICE guidelines recommend giving prophylactic oral nystatin to prevent fungal infections in babies treated with antibiotics for suspected late-onset neonatal infection, if they weigh under 1,500g or were born at less than 30 weeks gestation7. If it is not possible to administer oral nystatin, IV fluconazole can be used instead — this is an off-label use of fluconazole, so caution must be exercised when deciding what dose to give.
The South East London Paediatric Formulary recommends 3mg/kg every 72 hours for the first 2 weeks of life, then 3mg/kg every 48 hours for the following 2 weeks, then 3mg/kg every 24 hours for 2 weeks (until central IV line and endotracheal tubes have been removed)33. This changing dose interval reflects how preterm neonates are able to clear the drug faster as their liver enzymes mature34.
Prevention
Preventing infections in premature or unwell neonates is critical to reducing morbidity and mortality35. NICUs implement strict infection control measures, including maternal screening, antimicrobial prophylaxis and hygiene protocols35. These strategies are summarised in the Box 2.
Box 2: Maternal screening and intrapartum antibiotics
Pregnant women can be screened for group B strep and those who test positive should receive intrapartum antibiotic prophylaxis to reduce neonatal EOS. In the UK, routine universal screening for group B strep in pregnant women is not standard practice, unlike in countries such as the United States, Canada and Australia. Instead, the UK follows a risk-based approach to prevent early-onset group B strep infection in neonates36.
Antibiotics are offered to women in labour if they have:
- A previous baby affected by group B strep infection;
- Group B strep detected in urine or vaginal/rectal swabs during the current pregnancy;
- Preterm labour (<37 weeks);
- Prolonged rupture of membranes (>18 hours);
- Fever (≥38°C) during labour36.
This approach targets high-risk cases while avoiding unnecessary antibiotic exposure for low-risk pregnancies.
Maternal infections such as chorioamnionitis and urinary tract infections should be treated promptly to reduce the risk of vertical transmission37.
Strict hand hygiene and infection control measures
- Healthcare providers and caregivers must follow strict hand hygiene protocols before handling neonates38;
- Barrier precautions — such as gloves, gowns and masks — should be used where appropriate, especially for those with weakened immune systems39; however, studies have shown that good hand hygiene can be preferable to wearing gloves and the environmental impact should be considered40–42. Masks should be avoided where possible to allow babies to see facial expressions — to aid with their development43–45;
- Neonates with suspected infections are isolated to prevent cross-infection46.
Aseptic techniques for invasive procedures
- Sterile handling of catheters, IV lines and endotracheal tubes: Neonates frequently require central venous catheters, endotracheal tubes and feeding tubes, which increase infection risk if not handled aseptically47,48;
- Minimising unnecessary invasive procedures; this includes limiting blood draws, catheter use and intubations when not clinically necessary.
Breastfeeding and immune support
- Breast milk contains immunoglobulins, antimicrobial peptides and beneficial microbiota that protect against infections49. For preterm infants, donor human milk can potentially be used when maternal milk is unavailable50; however, not all hospitals have access to a human milk bank, leading to regional inequalities in donor milk availability51;
- Some studies suggest that probiotics can reduce the risk of NEC and late-onset sepsis in preterm neonates52.
Antibiotic stewardship
- Avoiding unnecessary antibiotic exposure to prevent antimicrobial resistance and gut microbiome disruption53,54. Empiric antibiotic therapy is initiated based on risk factors and clinical suspicion, but is discontinued if cultures are negative and clinical signs/symptoms have improved55.
Environmental infection control
- Regular cleaning and disinfection of NICU equipment, such as incubators, ventilators and monitors, to prevent pathogen transmission35.
Vaccination strategies
- Vaccinating mothers against influenza, pertussis, respiratory syncytial virus (RSV) and COVID-19 during pregnancy can provide passive immunity to neonates56.
Conclusion
Neonatal sepsis remains a leading cause of morbidity and mortality, particularly in preterm and critically ill infants57. Early recognition, timely antibiotic administration and appropriate antimicrobial stewardship are essential for optimal outcomes58. Pharmacists play a crucial role in ensuring correct antibiotic selection, dosing and monitoring to balance effective treatment with minimising resistance risks.
Best practice points for pharmacists
Antimicrobial stewardship — minimise resistance risks
- Review cultures at 36–48 hours to consider de-escalation;
- Stop antibiotics at 36 hours if sepsis is unlikely and cultures are negative;
- Use narrow-spectrum agents whenever possible;
- Avoid prolonged broad-spectrum antibiotics unless necessary.
Dosing and monitoring
- Adjust gentamicin (or amikacin) dosing based on gestational age and renal function;
- Monitor therapeutic drug levels (TDM) for gentamicin, amikacin and vancomycin;
- Ensure appropriate renal function monitoring (creatinine, urine output).
Prevention and risk reduction
- Promote maternal intrapartum antibiotic prophylaxis for group B strep prevention;
- Encourage hand hygiene and aseptic line management in NICUs;
- Support use of maternal breast milk and donor human milk where available to reduce NEC risk.
- 1.Fleischmann C, Reichert F, Cassini A, et al. Global incidence and mortality of neonatal sepsis: a systematic review and meta-analysis. Arch Dis Child. 2021;106(8):745-752. doi:10.1136/archdischild-2020-320217
- 2.Milton R, Gillespie D, Dyer C, et al. Neonatal sepsis and mortality in low-income and middle-income countries from a facility-based birth cohort: an international multisite prospective observational study. The Lancet Global Health. 2022;10(5):e661-e672. doi:10.1016/s2214-109x(22)00043-2
- 3.Ong WJ, Seng JJB, Yap B, et al. Impact of neonatal sepsis on neurocognitive outcomes: a systematic review and meta-analysis. BMC Pediatr. 2024;24(1). doi:10.1186/s12887-024-04977-8
- 4.Bell EF, Hintz SR, Hansen NI, et al. Mortality, In-Hospital Morbidity, Care Practices, and 2-Year Outcomes for Extremely Preterm Infants in the US, 2013-2018. JAMA. 2022;327(3):248. doi:10.1001/jama.2021.23580
- 5.Idzikowski E, Connors TJ. Impact and Clinical Implications of Prematurity on Adaptive Immune Development. Curr Pediatr Rep. 2020;8(4):194-201. doi:10.1007/s40124-020-00234-5
- 6.Tsafaras GP, Ntontsi P, Xanthou G. Advantages and Limitations of the Neonatal Immune System. Front Pediatr. 2020;8. doi:10.3389/fped.2020.00005
- 7.Neonatal infection – antibiotics for prevention and treatment. National Institute for Health and Care Excellence. 2021. Accessed April 2025. www.nice.org.uk/guidance/ng195
- 8.Goel N, Shrestha S, Smith R, et al. Screening for early onset neonatal sepsis: NICE guidance-based practice versus projected application of the Kaiser Permanente sepsis risk calculator in the UK population. Arch Dis Child Fetal Neonatal Ed. 2019;105(2):118-122. doi:10.1136/archdischild-2018-316777
- 9.Johnston N, de Waal K. Clinical and haemodynamic characteristics of preterm infants with early onset sepsis. J Paediatrics Child Health. 2022;58(12):2267-2272. doi:10.1111/jpc.16218
- 10.Newborn Early Warning Trigger and Track (NEWTT) – A Framework for Practice. British Association of Perinatal Medicine. 2023. Accessed April 2025. https://www.bapm.org/resources/deterioration-of-the-newborn-newtt-2-a-framework-for-practice
- 11.Neonatal Early-Onset Sepsis Calculator. Kaiser Permanente Research. Accessed April 2025. https://neonatalsepsiscalculator.kaiserpermanente.org/
- 12.Achten NB, Klingenberg C, Benitz WE, et al. Association of Use of the Neonatal Early-Onset Sepsis Calculator With Reduction in Antibiotic Therapy and Safety. JAMA Pediatr. 2019;173(11):1032. doi:10.1001/jamapediatrics.2019.2825
- 13.Puopolo KM, Lynfield R, Cummings JJ, et al. Management of Infants at Risk for Group B Streptococcal Disease. Pediatrics. 2019;144(2). doi:10.1542/peds.2019-1881
- 14.Benzylpenicillin sodium. British National Formulary for Children. Accessed April 2025. https://bnfc.nice.org.uk/drugs/benzylpenicillin-sodium/#indications-and-dose
- 15.Gentamicin. British National Formulary for Children. Accessed April 2025. https://bnfc.nice.org.uk/drugs/gentamicin/#indications-and-dose
- 16.Shang ZH, Wu YE, Lv DM, et al. Optimal dose of cefotaxime in neonates with early-onset sepsis: A developmental pharmacokinetic model-based evaluation. Front Pharmacol. 2022;13. doi:10.3389/fphar.2022.916253
- 17.Neonatal Listeriosis. MSD Manual Professional Edition. 2022. Accessed April 2025. https://www.msdmanuals.com/professional/pediatrics/infections-in-neonates/neonatal-listeriosis
- 18.Mukhopadhyay S, Puopolo KM. Risk Assessment in Neonatal Early Onset Sepsis. Seminars in Perinatology. 2012;36(6):408-415. doi:10.1053/j.semperi.2012.06.002
- 19.Song WS, Park HW, Oh MY, et al. Neonatal sepsis-causing bacterial pathogens and outcome of trends of their antimicrobial susceptibility a 20-year period at a neonatal intensive care unit. Clin Exp Pediatr. 2022;65(7):350-357. doi:10.3345/cep.2021.00668
- 20.Ericson JE, Agthe AG, Weitkamp JH. Late-Onset Sepsis. Clinics in Perinatology. 2025;52(1):33-45. doi:10.1016/j.clp.2024.10.003
- 21.Coggins SA, Glaser K. Updates in Late-Onset Sepsis: Risk Assessment, Therapy, and Outcomes. NeoReviews. 2022;23(11):738-755. doi:10.1542/neo.23-10-e738
- 22.Miselli F, Crestani S, Maugeri M, et al. Late-Onset Sepsis Mortality among Preterm Infants: Beyond Time to First Antibiotics. Microorganisms. 2023;11(2):396. doi:10.3390/microorganisms11020396
- 23.Gul A. Analysis of Late-onset Neonatal Sepsis Cases in a Level Three Neonatal Intensive Care Unit. North Clin Istanbul. Published online 2019. doi:10.14744/nci.2019.39018
- 24.Mir MA, Ashraf MW, Tripathi V, Mir BA. Isolation, characterization and prevention of various microbial strains in NIC unit and PIC unit. Sci Rep. 2021;11(1). doi:10.1038/s41598-020-79364-1
- 25.Hoffman A, Satyavolu S, Muhanna D, et al. Predictors of mortality and severe illness from Escherichia coli sepsis in neonates. J Perinatol. 2024;44(12):1816-1821. doi:10.1038/s41372-024-02117-9
- 26.Dermitzaki N, Baltogianni M, Tsekoura E, Giapros V. Invasive Candida Infections in Neonatal Intensive Care Units: Risk Factors and New Insights in Prevention. Pathogens. 2024;13(8):660. doi:10.3390/pathogens13080660
- 27.Downey LC, Smith PB, Benjamin DK Jr. Risk factors and prevention of late-onset sepsis in premature infants. Early Human Development. 2010;86(1):7-12. doi:10.1016/j.earlhumdev.2010.01.012
- 28.Özkavaklı A, Yalın İmamoğlu E, et al. Trends in Causative Organisms and Antimicrobial Resistance in Late-onset Neonatal Sepsis. Turkish Archives of Pediatrics. 2024;59(4):375-382. doi:10.5152/turkarchpediatr.2024.24006
- 29.Lutsar I, Chazallon C, Trafojer U, et al. Meropenem vs standard of care for treatment of neonatal late onset sepsis (NeoMero1): A randomised controlled trial. Scherag A, ed. PLoS ONE. 2020;15(3):e0229380. doi:10.1371/journal.pone.0229380
- 30.Wagstaff JS, Durrant RJ, Newman MG, et al. Antibiotic Treatment of Suspected and Confirmed Neonatal Sepsis Within 28 Days of Birth: A Retrospective Analysis. Front Pharmacol. 2019;10. doi:10.3389/fphar.2019.01191
- 31.Bersani I, Piersigilli F, Goffredo BM, et al. Antifungal Drugs for Invasive Candida Infections (ICI) in Neonates: Future Perspectives. Front Pediatr. 2019;7. doi:10.3389/fped.2019.00375
- 32.Kaufman DA, Mukhopadhyay S. Neonatal Invasive Fungal Infections. Clinics in Perinatology. 2025;52(1):47-66. doi:10.1016/j.clp.2024.10.004
- 33.The South East London Paediatric Formulary. The South East London Paediatric Formulary. Accessed April 2025. https://app.clinibee.com/articles/96f206ae-194d-4c66-96f7-568a7d7e353b
- 34.Turner K, Manzoni P, K. Benjamin D, Cohen-Wolkowiez M, B. Smith P, M. Laughon M. Fluconazole Pharmacokinetics and Safety in Premature Infants. CMC. 2012;19(27):4617-4620. doi:10.2174/092986712803306367
- 35.Johnson J, Akinboyo IC, Schaffzin JK. Infection Prevention in the Neonatal Intensive Care Unit. Clinics in Perinatology. 2021;48(2):413-429. doi:10.1016/j.clp.2021.03.011
- 36.Prevention of Early‐onset Neonatal Group B Streptococcal Disease. BJOG. 2017;124(12). doi:10.1111/1471-0528.14821
- 37.Conde-Agudelo A, Romero R, Jung EJ, Garcia Sánchez ÁJ. Management of clinical chorioamnionitis: an evidence-based approach. American Journal of Obstetrics and Gynecology. 2020;223(6):848-869. doi:10.1016/j.ajog.2020.09.044
- 38.Kuti BP, Ogunlesi TA, Oduwole O, et al. Hand hygiene for the prevention of infections in neonates. Cochrane Database of Systematic Reviews. 2023;2023(6). doi:10.1002/14651858.cd013326.pub4
- 39.Legeay C, Bourigault C, Lepelletier D, Zahar JR. Prevention of healthcare-associated infections in neonates: room for improvement. Journal of Hospital Infection. 2015;89(4):319-323. doi:10.1016/j.jhin.2015.02.003
- 40.Glove Awareness. Royal College of Nursing . Accessed April 2025. https://www.rcn.org.uk/Get-Involved/Campaign-with-us/Glove-awareness
- 41.Spooner R, Glover Williams A, Roome C. Improving the environmental sustainability of paediatric care. Arch Dis Child Educ Pract Ed. 2022;108(3):218-224. doi:10.1136/archdischild-2021-322933
- 42.Leonard A, Wilson N, Dunn H. 6 The gloves are off; safer in our hands. Changing glove use at an acute children’s trust. Abstracts. Published online November 2019:A2.3-A3. doi:10.1136/archdischild-2019-gosh.6
- 43.Green J, Staff L, Bromley P, Jones L, Petty J. The implications of face masks for babies and families during the COVID-19 pandemic: A discussion paper. Journal of Neonatal Nursing. 2021;27(1):21-25. doi:10.1016/j.jnn.2020.10.005
- 44.Burns KH, Saunders BS, Burns SA. Nurturing visual social development in the NICU. J Perinatol. 2020;41(8):2108-2109. doi:10.1038/s41372-020-00813-w
- 45.Carnevali L, Gui A, Jones EJH, Farroni T. Face Processing in Early Development: A Systematic Review of Behavioral Studies and Considerations in Times of COVID-19 Pandemic. Front Psychol. 2022;13. doi:10.3389/fpsyg.2022.778247
- 46.Hanna M, Shah R, Marquez L, Barzegar R, Gordon A, Pammi M. Infant isolation and cohorting for preventing or reducing transmission of healthcare-associated infections in neonatal units. Cochrane Database of Systematic Reviews. 2023;2023(6). doi:10.1002/14651858.cd012458.pub2
- 47.Cho HJ, Cho HK. Central line-associated bloodstream infections in neonates. Korean J Pediatr. 2019;62(3):79-84. doi:10.3345/kjp.2018.07003
- 48.Use of Central Venous Catheters in Neonates A Framework for Practice. British Association of Perinatal Medicine. December 2015. Accessed April 2025. https://hubble-live-assets.s3.eu-west-1.amazonaws.com/bapm/file_asset/file/60/BAPM_CVC_final_Jan16__addition_Aug_2018_.pdf
- 49.Carr LE, Virmani MD, Rosa F, et al. Role of Human Milk Bioactives on Infants’ Gut and Immune Health. Front Immunol. 2021;12. doi:10.3389/fimmu.2021.604080
- 50.Kashyap V, Choudhari SG. Unlocking the Potential: A Systematic Literature Review on the Impact of Donor Human Milk on Infant Health Outcomes. Cureus. Published online April 2, 2024. doi:10.7759/cureus.57440
- 51.Shenker NS, Griffin S, Hamill‐Keays J, Thomson M, Simpson J, Weaver G. Understanding the current and future usage of donor human milk in hospitals: An online survey of UK neonatal units. Maternal & Child Nutrition. 2023;19(4). doi:10.1111/mcn.13526
- 52.Alshaikh BN, Ting J, Lee S, et al. Effectiveness and Risks of Probiotics in Preterm Infants. Pediatrics. 2025;155(3). doi:10.1542/peds.2024-069102
- 53.Stocker M, Klingenberg C, Navér L, et al. Less is more: Antibiotics at the beginning of life. Nat Commun. 2023;14(1). doi:10.1038/s41467-023-38156-7
- 54.Cotten CM. Adverse consequences of neonatal antibiotic exposure. Current Opinion in Pediatrics. 2016;28(2):141-149. doi:10.1097/mop.0000000000000338
- 55.Gkentzi D, Dimitriou G. Antimicrobial Stewardship in the Neonatal Intensive Care Unit: An Update. CPR. 2019;15(1):47-52. doi:10.2174/1573396315666190118101953
- 56.Chittajallu LVS, Kaku R, Kondadasula P, Lim JY, Zhumabekova A. Safety and Efficacy of Vaccines During Pregnancy: A Systematic Review. Cureus. Published online January 9, 2025. doi:10.7759/cureus.77176
- 57.Attia Hussein Mahmoud H, Parekh R, Dhandibhotla S, et al. Insight Into Neonatal Sepsis: An Overview. Cureus. Published online September 19, 2023. doi:10.7759/cureus.45530
- 58.Sultan HM, Ibrahim AM, Elmahdy MAA. Neonatal sepsis: A review of current management strategies. Journal of Neonatal Nursing. 2024;30(6):539-551. doi:10.1016/j.jnn.2024.02.010