Science Photo Library
Summary
Morphoea and systemic sclerosis (SSc) are two forms of the autoimmune connective tissue disorder scleroderma. Lesions on the skin caused by morphoea will often resolve within a few years, but many patients may require treatment with topical corticosteroids, calcipotriol, topical tacrolimus or imiquimod. Intravenous or oral corticosteroids and other systemic immunosuppressing medicines may be required for deeper lesions.
SSc is a complex condition with many manifestations. Patients will often require treatment with a number of medicines. Management is centred on managing symptoms, preventing and treating complications and modifying disease progression.
[Also:
Scleroderma: clinical features and diagnosis
]
Scleroderma is an autoimmune disease that affects connective tissue in skin and internal organs. There are two distinct types of scleroderma: morphoea and systemic sclerosis (SSc) (See accompanying article ‘Scleroderma – clinical features and diagnosis’). The management of each condition should be considered separately.
Morphoea
There are no licensed interventions for the treatment of morphoea and there have been few controlled clinical trials. Most treatment regimens are therefore based on case reports. Most superficial lesions will resolve over three to five years and treatment may not be necessary, although progressive and deep lesions can be disabling and pharmacological therapy or phototherapy may be useful. Anecdotally, early treatment of newly formed lesions is more likely to be successful than treating those that are well established.
There are no clear guidelines on pharmacological treatment, and treatment is usually directed by a dermatologist. This usually involves the topical or intralesional application of a potent corticosteroid, which can control inflammation and may have a role in attenuating fibroblasts and other mesenchymal cells, especially in early lesions. Occasionally, topical calcipotriol will be used in combination. If long-term therapy is required to control, rather than treat, lesions, the use of corticosteroids should be minimised to prevent adverse effects.
There is some evidence to support the use of either tacrolimus 0.1% ointment or imiquimod 5% cream as a single treatment for morphoea[1]
.
Immunosuppression using oral or intravenous corticosteroids may be necessary to treat deeper lesions that have the potential to be disabling. Methotrexate is used in adults and children either as an alternative to, in conjunction with, or following treatment failure with topical corticosteroids. Mycophenolate mofetil can be used to treat patients who do not respond to methotrexate[2]
.
Patients who are disabled by bone or joint deformities or atrophy of subcutaneous tissue may also benefit from physiotherapy, occupational therapy and surgical interventions.
Systemic sclerosis
There is no cure for SSc and treatment focuses on providing symptomatic relief, managing complications and modifying disease progression.
Dermatological complications can be improved, although not resolved, by disease modifying therapies. Methotrexate has been shown to be effective at improving skin symptoms in early SSc[3]
.
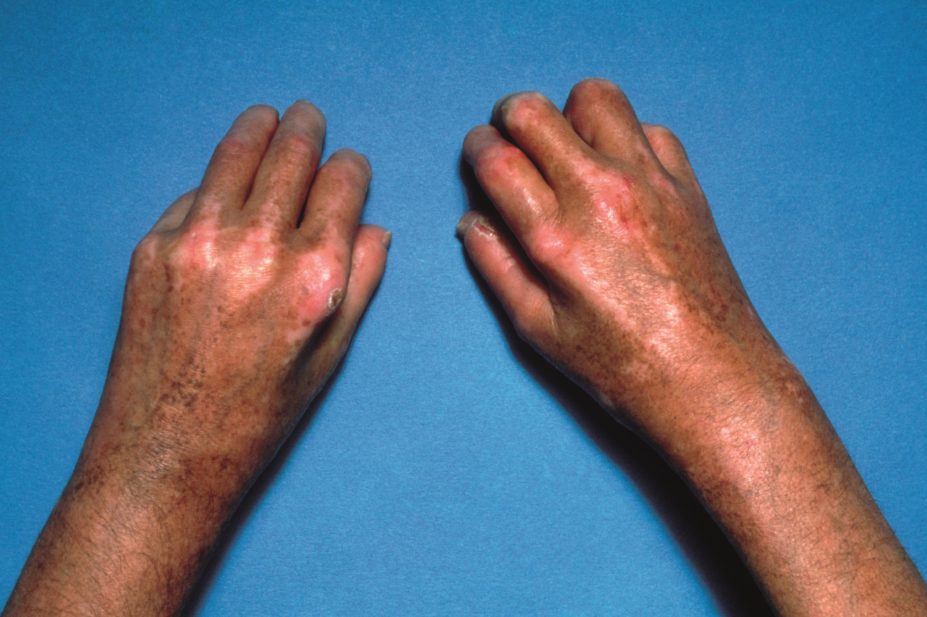
Tightening of the skin is a fundamental problem for patients with SSc, and hair and sweat glands will often be absent in affected areas. Common symptoms include skin contracture, dryness, irritation and pruritus, telangiectasia, and ulceration, which may be secondary to calcinosis. The Scleroderma Society provides comprehensive advice on self-management of skin symptoms of Ssc
[4]
. Management should focus on protection of the skin from irritation and injury, liberal use of emollients, and early treatment of ulceration or signs of skin and soft tissue infection.
Pruritus may respond to the liberal application of emollients, although the response is usually suboptimal. Antihistamines, tricyclic antidepressants and trazodone have all been used, but with varying degrees of success[5]
. There are no trials comparing treatments and
little consensus on what to use.
Telangiectasia, if upsetting for the patient, can be managed with cosmetic camouflage creams or laser treatment; the NHS does not routinely fund laser therapy for patients with SSc.
Disabling contractures can be treated with physiotherapy, occupational therapy or surgery.
There are no evidence-based pharmacological treatments to prevent or treat calcinosis. Surgical removal is an option if calcium deposits cause discomfort, are of a cosmetic concern or result in ulceration. Calcium deposits can often recur in patients with SSc.
Raynaud’s phenomenon, associated with SSc, can be managed with advice to reduce the frequency of ischaemic attacks, such as staying warm and avoiding touching cold surfaces. Hand exercises can help maintain blood flow and may also improve skin flexibility. Patients should avoid doing anything that could restrict circulation of blood to the fingers, such as carrying shopping bags by hand (no matter how brief the activity).
If treatment is required, dihydropyridine-type calcium channel blockers (usually nifedipine, but also amlodipine) are considered first-line for SSc-associated Raynaud’s phenomenon by the European League Against Rheumatology (EULAR) and Scleroderma Trials And Research Group (EUSTAR), and have been proven to reduce the incidence of ischaemic attacks[3]
. Intravenous iloprost — administered daily for 3–5 days every 6–8 weeks — has been found to be slightly more efficacious than nifedipine (30–60mg daily). EULAR recommends iloprost is used for severe SSc-associated Raynaud’s phenomenon where calcium antagonists have been unsuccessful[3]
. Intravenous iloprost is available on a named patient basis.
There is limited evidence to suggest the peripheral vasodilatory effect of the serotonin reuptake inhibitor fluoxetine can be of benefit for some patients[6]
.
Xerophtalmia (dry eyes) and xerostomia (dry mouth) can be managed using saliva substitutes and ocular lubricants.
Managing complications
Digital ischaemia and subsequent ulceration is a possible complication of SSc. Treatments that improve digital circulation can prevent ulcers from forming and speed healing of established ulceration.
Intravenous iloprost is used for the treatment of active digital ulcers for patients with SSc, although its place in therapy depends on the severity of the digital ischaemia and the frequency of attacks[3]
.
Intravenous epoprostenol, which is occasionally used in SSc-associated pulmonary arterial hypertension, has been shown to reduce the number of new digital ulcers by around 50%[3]
. It is not licensed for this indication and would generally only be used if indicated for the treatment of pulmonary arterial hypertension associated with SSc.
Bosentan, an endothelin receptor antagonist, is licensed to reduce the incidence of new ulcers in patients with multiple digital ulcers and in whom calcium antagonists and prostanoids have failed to have an effect. It has also been shown to improve healing of established ulcers[3]
. It is prescribed at a dose of 62.5mg twice a day for four weeks increasing to 125mg twice a day.
Bosentan is associated with hepatotoxicity and teratogenicity. Liver function tests are required monthly throughout treatment and two weeks after any dose increases. Women of childbearing age are advised to take monthly pregnancy tests to allow early detection of pregnancy. Bosentan is both an inducer and a substrate for the isoenzymes CYP2C9 and CYP3A4 and is, therefore, associated with numerous interactions, including a reduction in the efficacy of hormonal contraception.
Pulmonary hypertension is an important respiratory complication of SSc associated with a high mortality rate and its management is outside the scope of this article. Both pulmonary arterial hypertension (PAH) and pulmonary hypertension (PH) can be seen in SSc. These conditions are not synonymous and targeted PAH therapies may worsen symptoms when used inappropriately for patients with PH. SSc is the second most common cause of PAH and is the only condition where yearly screening for PH is recommended.
Interstitial lung disease is associated with SSc, causing inflammation and pulmonary fibrosis, which can lead to respiratory failure. Cyclophosphamide, mycophenolate mofetil and rituximab have been studied for the management of interstitial lung disease in patients with SSc.
Oral mycophenolate mofetil has also been shown to improve lung function in patients with SSc-associated connective tissue disorder-interstitial lung disease[7],[8]
.
Oral cyclophosphamide (1–2mg/kg daily) has been found to improve lung function tests, dyspnoea and quality of life over 12 months in patients with SSc[9]
. Intravenous cyclophosphamide 600mg/m2 monthly for six months followed by oral azathioprine 2.5mg/kg daily and prednisolone 20mg every other day has also been shown to improve lung function.
A recent systematic review focused on improvement in forced vital capacity (FVC) and diffusing capacity of the lung for carbon monoxide (DLCO) found that treatment with cyclophosphamide was not beneficial in SSc [10]
. However, the main aim of treatment is not improvement but prevention of disease progression, which is difficult to assess in studies due to the variability of progression in patients before treatment begins.
There are no clear guidelines on who to treat with cyclophosphamide, although it is typically used in patients with rapidly progressing respiratory symptoms, patients with severe functional impairment, or patients whose symptoms continue to progress despite immunomodulatory treatment with mycophenolate. Cyclophosphamide is cytotoxic and associated with a number of adverse effects (see ‘Adverse effects of cyclophosphamide’).
Adverse effects of cyclophosphamide
Acrolein, a toxic metabolite of cyclophosphamide, is renally excreted and can cause drug-induced haemorrhagic cystitis and malignancy in the long term. Patients should ensure adequate hydration during treatment to ensure dilution and clearance of the metabolite. Mesna chemically interacts with and inactivates acrolein; it is often given to SSc patients receiving cyclophosphamide. A number of different regimens are used, which can include either or both IV and oral doses.
Treatment with cyclophosphamide can also cause ovarian failure in women and infertility in men. The risk of ovarian failure seems to be related to age and number of doses received. Patients should be made aware of these risks. If treatment is essential, sperm- or egg-banking should be considered.
Gonadotropin-releasing hormone agonists, such as goserelin and triptorelin, are often used to protect ovarian function in premenopausal women treated with cyclophosphamide. However, there is limited evidence to support this practice.
The monoclonal antibody rituximab has been shown to prevent worsening lung fibrosis, as demonstrated in the recent EUSTAR study[11]
. The RECITAL trial, which will compare rituximab and cyclophosphamide, is currently recruiting patients.
Patients with pulmonary hypertension and interstitial lung disease often require oxygen therapy. It is recommended when there is evidence of symptomatic benefit and correctable desaturation on exercise. Because of the irreversible and progressive nature of lung involvement in SSc, lung transplantation may be considered in some patients with end-stage lung disease or cardiopulmonary failure.
Cardiovascular disease in patients with SSc requires careful management. Beta-blockers are contraindicated, because there is a risk of worsening peripheral ischaemia, and antiplatelets may further increase the risk of a GI bleed. Some patients may also have myositis, either because of autoimmune inflammation associated with SSc or as a result of ongoing tissue ischaemia. These patients may be at increased risk of muscle toxicity if statins are used.
Gastrointestinal complications, such as gastro-oesophageal reflux disease, oesophageal ulcers and strictures, and symptomatic motility disturbances, can be managed with proton pump inhibitors and prokinetics. However, the evidence to support their use in patients with SSc is limited. Laxatives are often used to prevent faecal loading and overflow incontinence. Octreotide and prucalopride can be used to manage pseudo-obstruction[12]
.
Scleroderma renal crisis that is not promptly managed often leads to renal failure, requiring dialysis or renal transplant. Although there is a lack of clinical research to identify strategies to prevent this complication, there are numerous case series reports that support the routine use of angiointensin-converting enzyme (ACE) inhibitors to increase survival. Most specialists now consider ACE inhibitors to be essential in the management of SSc.
Corticosteroids have been shown, in some case series, to increase the risk of renal crisis. It is difficult to prove this is a cause, but most experts recommend caution when considering corticosteroid use for patients with SSc[13],[14]
.
For patients in renal crisis, the aim of treatment is to bring blood pressure down at a rate of 20mmHg (systolic) and 10mmHg (diastolic) per day to normal values, taking care to avoid prolonged periods of hypotension. There is a lack of consensus on an appropriate method to achieve this; however, intravenous iloprost, ACE inhibitors, angiotensin receptor antagonists, calcium channel blockers and alpha blockers — used alone and in conjunction — have all been used[14]
.
[Also:
Scleroderma: clinical features and diagnosis
]
Author details
Lloyd Mayers, ClinDipPharm, MRPharmS, is a specialist pharmacist at North Bristol NHS Trust. Patricia Ging BPharm, MPSI, M.Sc is a transplant and pulmonary hypertension pharmacist at Mater Misericordiae University Hospital in Dublin, Ireland.
References
[1] Kroft EBM, Groeneveld TJ, Seyger MMB et al. Efficacy of topical tacrolimus 0.1% in active plaque morphea: randomized, double-blind, emollient-controlled pilot study. Am J Clin Dermatol 2009;10:181–187.
[2] Fett N, Werth VP. Update on morphea: part II. Outcome measures and treatment. J Am Acad Dermatol 2011;64:231–242.
[3] Kowal-Bielecka O, Landewé R, Avouac J et al. EULAR recommendations for the treatment of systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group (EUSTAR). Ann Rheum Dis 2009;68:620–628.
[4] Scleroderma Society. The Skin in Scleroderma [Online]. Available from www.sclerodermasociety.co.uk
[5] Frech TM & Baron M. Understanding itch in systemic sclerosis in order to improve patient quality of life. Clin Exp Rheumatol 2013;31(2):81–88.
[6] Coleiro B, Marshall SE, Denton CP et al. Treatment of Raynaud’s phenomenon with the selective serotonin reuptake inhibitor fluoxetine. Rheumatology (Oxford) 2001;40(9):1038–1043.
[7] Mendoza FA, Nagle SJ, Lee JB et al. A prospective observational study of mycophenolate mofetil treatment in progressive diffuse cutaneous systemic sclerosis of recent onset. J Rheumatol 2012;39(6):1241–1247.
[8] Fraiser LH, Kanekal S & Kehrer JP. Cyclophosphamide toxicity. Characterising and avoiding the problem. Drugs 1991;42:781–795.
[9] Tashkin DP1, Elashoff R & Clements PJ. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med 2006 22;354:2655–2666.
[10] Poormoghim H, Moradi Lakeh M, Mohammadipour M et al. Cyclophosphamide for scleroderma lung disease: a systematic review and meta-analysis. Rheumatol Int 2012; 32:2431–2444
[11] Jordan S, Distler JHW, Maurer B et al. Effects and safety of rituximab in systemic sclerosis: an analysis from the European Scleroderma Trial and Research (EUSTAR) group. Ann Rheum Dis 2014. doi:10.1136/annrheumdis-2013-204522.
[12] Ebert EC. Gastric and enteric involvement in progressive systemic sclerosis. J Clin Gastroenterol 2008;42(1):5–12.
[13] Trang G, Steele R, Baron M et al. Corticosteroids and the risk of scleroderma renal crisis: a systematic review. Rheumatol Int 2012;32(3):645–653.
[14] Penn H & Denton CP. Diagnosis, management and prevention of scleroderma renal disease. Curr Opin Rheumatol 2008;20(6):692–696.