Shutterstock.com
A one-off treatment for babies with a rare genetic disorder is the first gene therapy to be accepted by the Scottish Medicines Consortium (SMC) for use by NHS Scotland, and could become the most expensive treatment to ever be approved by the National Institute for Health and Care Excellence (NICE) in England and Wales.
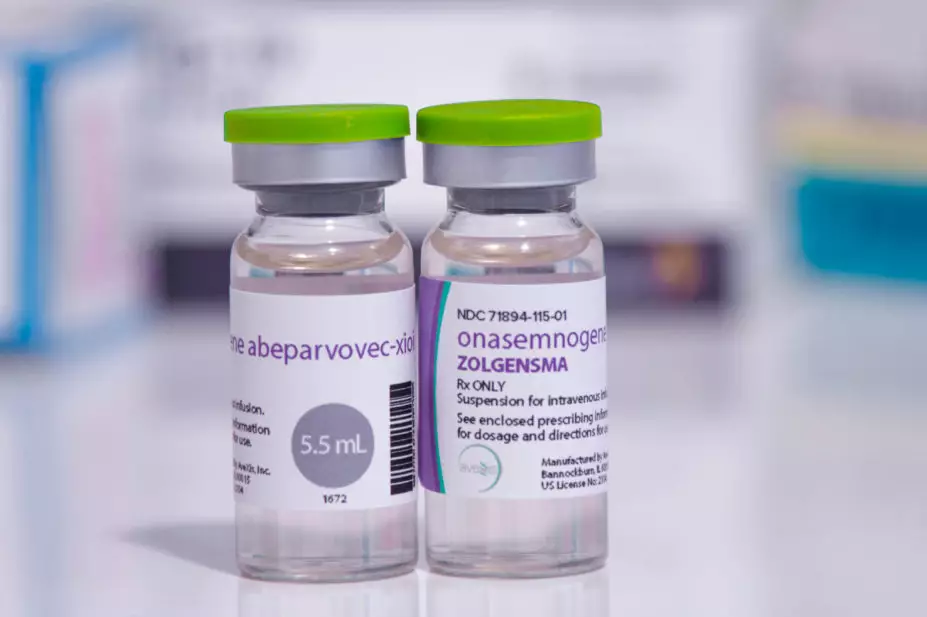
Onasemnogene abeparvovec (Zolgensma; Novartis Gene Therapies), which costs £1.79 million per dose, is a potentially curative therapy for babies with spinal muscular atrophy (SMA); a rare, progressive neuromuscular condition caused by a genetic mutation in the SMN1 gene on chromosome 5q.
SMA, which affects around 65 babies born in England each year, affects the nerves in the spinal cord controlling movement, causing muscle weakness, progressive loss of movement, and difficulties with breathing and swallowing.
In draft guidance, published on 8 March 2021, NICE recommends use of the drug in babies aged up to 12 months with type 1 SMA — one of the most severe forms of the condition, occurring in around 60% of SMA cases.
NICE also recommends its use in pre-symptomatic babies with SMA who have up to three copies of the SMN2 gene, as part of a managed access arrangement while further data is collected.
According to NICE, despite the high cost of the treatment, it can be recommended for use on the NHS because of the evidence of exceptional benefit to young babies, potentially allowing them to reach normal childhood developmental milestones; left untreated, the life expectancy in babies with type 1 SMA is around two years.
The drug would be provided at a discount on its list price to the NHS, but the actual price the NHS would pay has not been revealed for commercial reasons.
Meindert Boysen, deputy chief executive and director of the Centre for Health Technology Evaluation at NICE, said the committee concluded that onasemnogene abeparvovec represented an important development in treating SMA. It could allow babies to gain important motor milestones such as independent sitting and walking, and for some babies who are diagnosed before they have symptoms, it might come close to being a cure.
“As is the case with many new treatments for very rare diseases, limited evidence means there are uncertainties about the long-term benefits of onasemnogene abeparvovec,” he said.
“The collaborative effort of all involved in the evaluation has made it possible to recommend a treatment which, at its list price, is the most expensive drug NICE has ever evaluated, allowing the lives of babies who might otherwise have died before their second birthday to be transformed.”
Mark MacGregor, chair of the SMC, said that the decision to accept the drug was “incredibly difficult”.
“This medicine has the potential to be life changing for patients and their families. However, it is extremely expensive for the single dose required, even with the Patient Access Scheme discount offered by the company,” he said.
Onasemnogene abeparvovec will become the second gene therapy recommended by NICE that uses the adeno-associated virus (AAV) vector. In this case, the AAV vector is modified to carry a functional copy of the SMN1 gene into the motor neurons with the aim of promoting their survival and function. It is given as a one-off infusion and its effects are thought to be life-long.
The treatment was considered under the NICE Highly Specialised Technologies programme, which uses a higher threshold for cost effectiveness to reflect the difficulties of developing treatments for extremely rare conditions that might only affect a handful of patients per year.
NICE currently recommends the disease-modifying therapy nusinersen for some people with pre-symptomatic SMA and types 1, 2 or 3 SMA as part of a managed access agreement.
The NICE draft guidance is open for consultation until 6 April 2021.