Shutterstock.com
The National Institute for Health and Care Excellence (NICE) has confirmed draft guidance recommending the use of semaglutide, an appetite suppressant, for overweight and obese adults in the UK.
The guidance, first published in draft form on 8 February 2022, recommends that semaglutide (Wegovy; Novo Nordisk) should be funded by the NHS and used as part of a specialist weight management service for a maximum of two years.
Based on evidence from the ‘Semaglutide Treatment Effect in People with Obesity’ (STEP) 1 randomised controlled trial, the guidance recommends that semaglutide should be considered in adults with at least one weight-related condition and a body mass index (BMI) of at least 35. Combined with diet and lifestyle changes, it has been shown to help those using it reduce their weight by more than 10 per cent.
According to the newly confirmed recommendations, NICE suggests the drug should be prescribed to people within tier 3 and tier 4 of specialist multidisciplinary weight management programmes.
The guidance also states that the BMI threshold for access to the drug should be reduced, using lower BMI thresholds for patients from south Asian, Chinese, and black African or Caribbean family backgrounds following recommendations in NICE’s guideline on preventing ill-health and premature death in black, Asian and other minority ethnic groups.
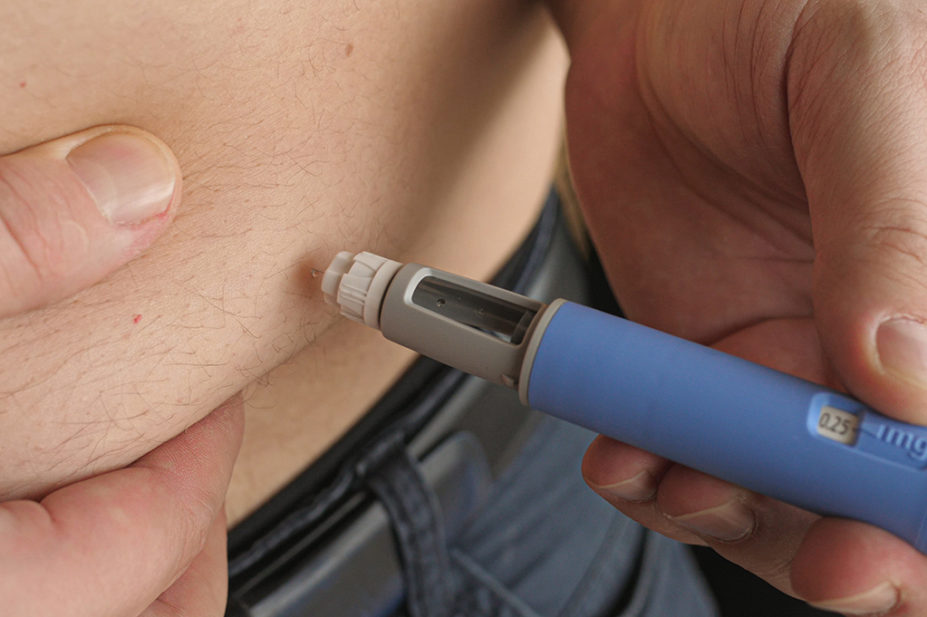
Semaglutide acts as an appetite suppressant by mimicking the hormone glucagon-like peptide-1 (GLP-1) released naturally after eating. By self-injecting semaglutide using weekly pre-filled pens, patients feel fuller and consequently consume fewer calories, leading to weight loss.
Helen Knight, programme director at the centre for health technology evaluation at NICE, described obesity as “one of the biggest challenges our health service is facing” and stated that NICE is now in a position to recommend a new line of pharmaceutical treatments to tackle the obesity epidemic.
Hannah Beba, consultant diabetes pharmacist for NHS Leeds Clinical Commissioning Group, described NICE’s recommendation as “a huge game changer”.
“People are already chomping at the bit to use this medication, so it’s excellent that finally we’re moving forward,” she said.
Obesity management is a crucial area of population health management, she said, and using a drug with a much broader licence could make a real difference.
“It’s been a very popular drug,” she said.
“The main concern is how weight management services are going to actually cope with the demand of these sorts of medications, so there’s some system redesign that needs to happen around this as well.”