Shutterstock.com
The National Institute for Health and Care Excellence (NICE) has recommended that a weekly injection to aid weight loss in overweight and obese adults should be funded on the NHS.
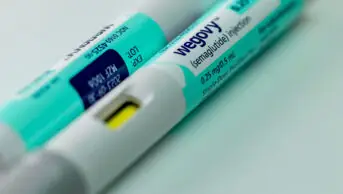
In draft guidance, published on 8 February 2022, NICE recommends semaglutide (Wegovy; Novo Nordisk) — which is already indicated for the treatment of type 2 diabetes mellitus — should be considered in adults with at least one weight-related condition and a body mass index (BMI) of at least 35.
It also recommends semaglutide “exceptionally” to people with a BMI of 30.0kg/m2 to 34.9kg/m2, if they are referred to tier 3 services based on the criteria in NICE’s obesity guideline.
A lower BMI threshold (usually reduced by 2.5kg/m2) has been recommended for people from south Asian, Chinese, and Black African or Caribbean family backgrounds, following recommendations in NICE’s guideline on preventing ill health and premature death in black, Asian and other minority ethnic groups.
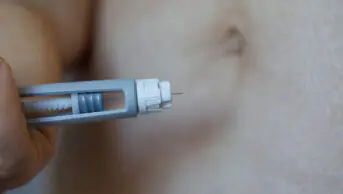
Semaglutide, which is injected by patients themselves once a week with pre-filled pens, suppresses the appetite by mimicking the hormone glucagon-like peptide-1 (GLP-1), which is released after eating. This makes the user feel full, leading to a reduction in overall calorie intake.
According to NICE, the drug can only be prescribed as part of a specialist weight management service with multidisciplinary input — such as a tier 3 weight management programme or tier 4 specialist obesity services, including surgery service — and for a maximum of two years.
The recommendation is based on evidence from the randomised controlled trial ‘Semaglutide Treatment Effect in People with Obesity’ (STEP) 1, which found that participants taking semaglutide lost, on average, 12% more of their body weight compared with placebo.
It comes after NICE recommended another GLP-1 receptor agonist, liraglutide (Saxenda; Novo Nordisk), in December 2020, as an option for managing overweight and obesity, alongside a reduced-calorie diet and increased physical activity, in adults with a BMI over 35.
Helen Knight, programme director at the centre for health technology evaluation at NICE, said: “We know that management of overweight and obesity is one of the biggest challenges our health service is facing, with nearly two thirds of adults either overweight or obese.
“But, in recent years, NICE has been able to recommend a new line of pharmaceutical treatments, which have shown that those people using them, alongside changes to their diet and exercise, have been able to reduce their weight.”
Hannah Beba, consultant diabetes pharmacist for NHS Leeds Clinical Commissioning Group, said the release of the draft guidance was “welcomed” owing to the “paucity” of medications for the management of obesity.
“Semaglutide has shown itself to be highly efficacious alongside diet and lifestyle changes,” she said.
“The STEP 1 trial showed up to 14.9% weight loss in individuals on semaglutide versus 2.4% on placebo.”
Beba said that the recognition in the inclusion criteria that obesity risks were seen at lower BMIs in people of south Asian, Chinese and Black African or Caribbean family backgrounds was “a great step forward”.
However, she added that the suggested placement of access to the medication within tier 3 and 4 services was “slightly disappointing”.
“There is emphasis from NICE on the importance of the multidisciplinary weight management teams at tier 3 and 4 that enable the cost-effective use of this medication, but it does make us take stock of if this is an inward facing approach and if access, as with many things, will be inadvertently restricted to people who are more able and willing to attend secondary care settings.”
The draft guidance will be out for consultation until 1 March 2022.
Read more: Semaglutide effective for weight loss in non-diabetic adults, research suggests