Shutterstock.com
The National Institute for Health and Care Excellence (NICE) has recommended the first drug for the treatment of severe hair loss owing to alopecia areata in people aged 12 years and over.
Ritlecitinib (Litfulo; Pfizer), a kinase inhibitor, is a once-daily oral pill. It works by reducing the enzymes that cause inflammation and subsequent hair loss at the follicle.
In a statement published alongside its final draft guidance on 22 February 2024, NICE said it estimated up to 14,000 people in England would benefit from the treatment, reversing their initial decision against use of the drug in September 2023.
In the statement, NICE said ritlecitinib was not recommended by its independent appraisal committee in September 2023, but the committee was able to make a positive recommendation on the new treatment following “the company [manufacturer] providing additional information and an improved discount to its price” after the initial consultation.
Evidence from clinical trials showed that ritlecitinib is more effective than placebo at improving hair regrowth and that response rates continued to improve for people taking the drug for up to two years.
Commenting on the recommendation, Helen Knight, director of medicines evaluation at NICE, said: “Our committee heard how severe alopecia areata can have a significant impact on people’s health and quality of life.
“I’m delighted that we are now able to recommend this innovative treatment, the first time a medicine for severe alopecia areata has been recommended by NICE for use in the NHS.
“It is especially pleasing that we have been able to recommend ritlecitinib just 16 weeks after it was granted a licence by the Medicines and Healthcare products Regulatory Authority, demonstrating NICE’s commitment to getting the best care to patients fast,” she added.
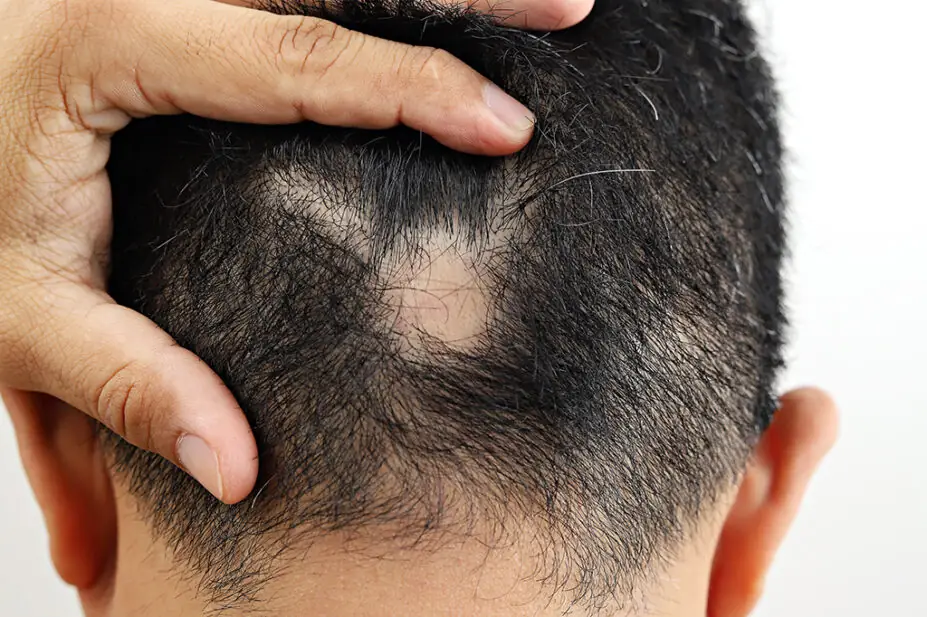
Alopecia areata is an autoimmune disease, where the body’s immune system attacks the hair follicles causing the hair to fall out.
It commonly affects the scalp but can also affect the eyelashes and eyebrows, nasal hair and hair on the skin, leaving people more vulnerable to infections and reducing their ability to regulate their body temperature.
Commenting on the announcement, Sue Schilling, chief executive of Alopecia UK, said: “This is a monumental day for the alopecia areata community.
“For far too long, patients with alopecia areata have gone without a licensed treatment option available via NHS pathways. This latest NICE recommendation will go some way to address this.”
“Our community still faces substantial barriers, including difficulties in getting a dermatology referral from their GP, unacceptable dermatology waiting times, and even some NHS trusts making the decision not to allow dermatology appointments for alopecia patients,” she said.
“There is no longer the excuse of there being no licensed treatment available. I urge key decision makers within the NHS to keep referral pathways open for patients with alopecia areata.”
The final NICE guidance is expected to be published on 27 March 2024.