Shutterstock.com
The UK government has said it expects all NHS and private healthcare providers to follow national guidance to restrict use of semaglutide in patients with type 2 diabetes mellitus (T2DM), amid ongoing shortages.
The Department of Health and Social Care (DHSC) issued a national patient safety alert on 18 July 2023, which advises providers to proactively identify all patients established on treatment with glucagon-like peptide-1 receptor agonists (GLP-1 RAs) to discuss stopping treatment if they had not achieved targets set out in National Institute for Health and Care Excellence (NICE) guidance.
The alert also warns not to switch between brands of GLP-1 RAs “including between injectable and oral forms” and not to double up on lower-dose preparations when the higher dose is unavailable.
Prescribers were previously warned in a medicine supply notification issued by the DHSC on 28 June 2023 to avoid initiating use GLP-1 RAs for T2DM patients and to only use the drugs for their licensed indication. Semaglutide, a GLP-1 RA, has been approved by NICE to help reduce weight, although the format of the drug approved for weight loss treatment is not yet available in the UK.
The latest national patient safety alert says there are “very limited, intermittent supplies” of all GLP-1 RAs and supplies are not expected to stabilise until at least mid-2024.
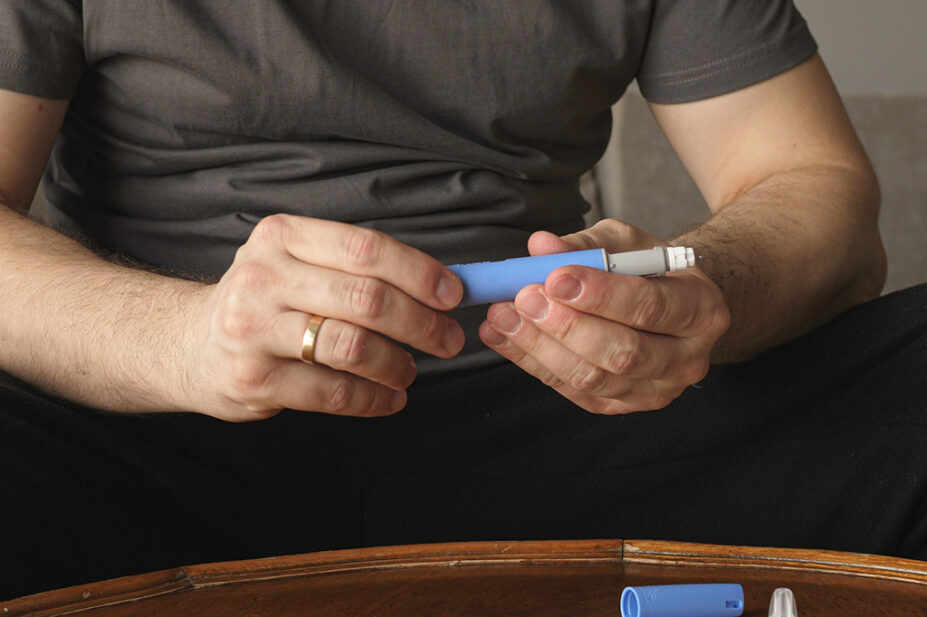
The drugs impacted by shortages include: semaglutide (Ozempic; Rybelsus); dulaglutide (Trulicity); liraglutide (Saxenda; Victoza) and exenatide (Byetta; Bydureon).
The alert attributed the shortages to “an increase in demand for these products for licensed and off-label indications”.
“The off-label use of these agents for the management of obesity is strongly discouraged. Existing stock must be conserved for use in patients with diabetes,” it said.
Some patients already established on GLP-1 RA therapy for T2DM may need to be switched to alternative treatments, such as insulin, the alert warned.
“Initiating insulin therapy requires training and education alongside a potential need for enhanced glucose monitoring to ensure patients are aware of how to recognise and manage hypoglycaemic events,” it said.
Some pharmacists have criticised the ongoing availability of semaglutide for weight loss through online providers.
A spokesperson for the DHSC said: “We expect all providers of healthcare services, whether NHS or private, and all those with responsibility for prescribing to take appropriate account of national guidance, such as national patient safety alerts and medicine supply notifications.
“The guidance is clear that these medicines should only be prescribed for the treatment of T2DM, in order to protect supply for diabetes patients.
“Medicines which are solely licensed to treat T2DM should not be routinely prescribed for weight loss.”
1 comment
You must be logged in to post a comment.


In a world where we accept obesity to be a leading risk factor for cardiovascular disease and t2dm and many other conditions, why is the use of GLP1 agonists for weight loss use being criticised? I think it’s shortsighted. Patients with obesity who feel the need to pay privately to access drugs that could help them avoid long term conditions (not to mention improved mental health) are a massive cost-saving for the NHS and should not be made to feel that they are depriving other people of medication.