Shutterstock.com
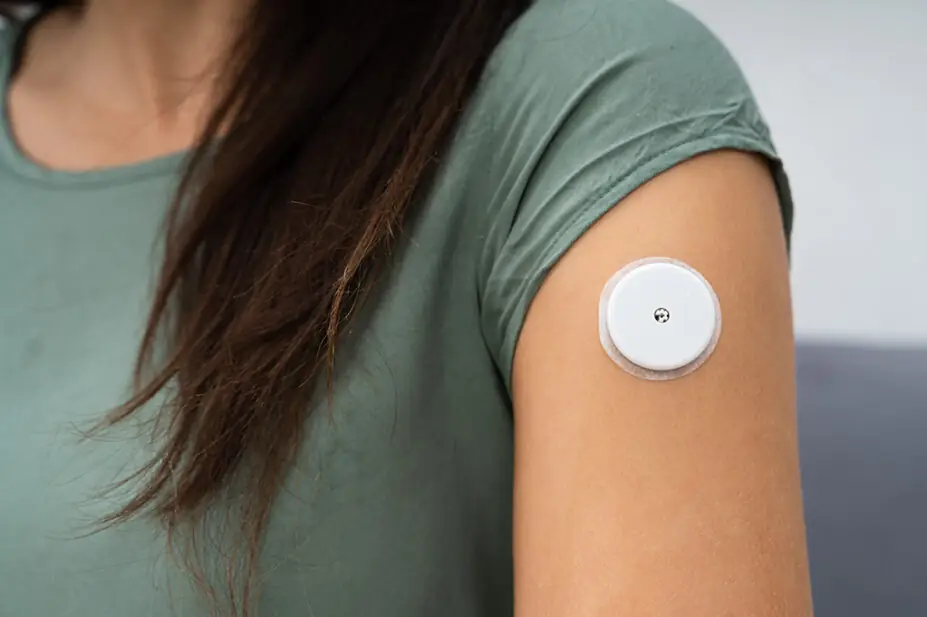
The Medicines and Healthcare products Regulatory Agency (MHRA) has indicated that safety problems in continuous glucose monitors (CGMs) and insulin pumps are potentially being underreported.
In a statement published on 8 October 2024, the MHRA said that it had received fewer than 300 Yellow Card reports relating to the devices from healthcare professionals and members of the public, which it said was “significantly fewer than we would expect, given their widespread use”.
In an email to The Pharmaceutical Journal, the MHRA clarified that it had received 273 reports since January 2023.
“In that same time period, we have additionally received greater numbers of reports from the manufacturers of these devices,” the email added.
“It is important to note that some of these reports relate to the diabetes management system as a whole and hence not all the reports are related to the insulin pump or the continuous glucose monitor.
“However, the receipt of these reports from manufacturers helps to highlight that the MHRA is not receiving as many reports direct from users as we would expect.”
The MHRA uses the Yellow Card reporting scheme to identify safety concerns that may require action.
In its announcement, the MHRA said it has published guidance for individuals living with diabetes on how to report safety concerns with diabetes management equipment to the scheme.
It also urged people to speak to a healthcare professional if they have concerns that their health may have been impacted by a potential safety issue relating to their device.
Pharmacists in England can complete a Yellow Card report online on the Yellow Card website or via the Yellow Card app.
While most adverse incidents relating to the devices do not result in harm to the patient, they could lead to the incorrect amount of insulin, which can lead to abnormal blood sugar levels and potentially serious health consequences.
Examples of the types of issue with CGMs and insulin pumps that should be reported include: concerns with accuracy of delivery from the insulin pump (e.g. suspected underdose or overdose, unexpected bolus doses, non-delivery of insulin); concerns with accuracy of results from a CGM; and skin reaction to the sensor adhesive.
In 2022, the National Institute for Health and Care Excellence recommended the use of real-time continuous glucose monitoring for adults and children living with type 1 diabetes mellitus for the first time.
Alison Cave, chief safety officer at the MHRA, said: “We know adverse incidents can occur with the use of these devices. The vast majority of these incidents don’t result in harm but potentially could have serious consequences.
“Every report is valuable to us as it will provide valuable insight and potentially inform future regulatory measures designed to protect patients. We are ready to take whatever action is needed.”
Douglas Twenefour, head of care at Diabetes UK, said: “Diabetes technology can be a life-changing tool, helping people living with the condition improve their quality of life.
“Unfortunately, we know that sometimes this technology doesn’t work as intended, so it is important that users of diabetes tech have a clear and accessible way to report any issues with continuous glucose monitors, insulin pumps and pens.”
Partha Kar, type 1 diabetes mellitus and technology lead for NHS England, added: “We welcome this work and its important role in ensuring safety while we oversee the widespread adoption of diabetes technologies using continuous glucose monitors and insulin pumps.
“These devices can be life-changing for people living with diabetes, giving them the confidence to go about their days knowing they are safe and able to enjoy themselves, so their [operational] effectiveness is of paramount importance.
“This initiative will help to ensure standards stay at the highest level as the market continues to expand with new developers.”