Abstract
Chronic kidney disease is a growing public health issue, with underdiagnoses and under treatment being important areas for improvement. The burden of chronic kidney disease disproportionately affects disadvantaged communities. Early identification and timely intervention are critical to slowing kidney disease progression, reducing cardiovascular complications and improving outcomes. While effective treatments exist, such as blood pressure control, lipid lowering therapy, sodium-glucose cotransporter 2 inhibitors and finerenone, there remains a gap in patients receiving optimal management.
This paper discusses the adoption of novel diagnostic tools, risk stratification methods and integrated care models that prioritise proactive, patient-centred approaches. By advancing co-designed patient education initiatives, closing treatment gaps and enhancing data-driven decision-making, kidney disease care can be transformed and its long-term burden reduced on individuals, communities and the healthcare system.
Introduction
Chronic kidney disease (CKD) affects over 700 million people globally1. In recognition of its growing impact, the World Health Organization adopted a global resolution in February 2025, recognising CKD as a significant public health issue and urging countries to strengthen prevention, early detection and integrated care efforts2.
CKD is defined as abnormalities of kidney structure or function, which are present for a minimum of three months, with implications for health3. CKD leads to several comorbidities, increasing the risk of adverse outcomes, including worsening kidney function and end-stage kidney disease (ESKD). Cardiovascular disease (CVD) is the leading cause of death in those with CKD4,5. It is estimated that 7.2 million individuals in the UK are currently living with CKD, with estimated NHS costs of £6.4bn annually6 . If current trends continue, this will increase to 7.6 million people, with the financial burden reaching £10.9bn by 20336. Beyond costs, CKD can have a profound impact on quality of life, with patients that require dialysis reporting significant limitations in mobility, daily activities and mental health6.
Investment into early identification strategies that focus on case finding, optimising prevention methods and treatment, and creating sustainable clinical pathways to improve access to services is essential to make a significant difference for patients and their healthy life expectancies.
This article will cover the adoption of novel diagnostic tools, risk stratification methods and integrated care models, primarily in England, that prioritise proactive, patient-centred approaches, as well as how CKD-related health inequalities can be addressed.
Classification and risk stratification
CKD is classified by the cause, estimated glomerular filtration rate (GFR) stage (G1-G5) and degree of albuminuria (A1-A3), which is referred to as CGA3 .
When the glomerulus of the kidney has an abnormally high permeability, which is a sign of kidney damage, albumin leaks into the urine7. This is known as albuminuria and is the most clinically relevant form of proteinuria in CKD. GFR indicates how effectively the kidneys are filtering the estimated waste in the blood7. In order to diagnose CKD, the functional markers required include a sustained estimated GFR (eGFR) <60mL/min/1.73m2 and/or albuminuria (i.e. a urinary albumin:creatinine ratio [uACR] of > 3mg/mmol) on a minimum of two occasions, three months apart8. Structural or histological abnormalities, such as haematuria or tubular disorders that lead to electrolyte imbalances, may also indicate CKD8. To standardise risk stratification, the National Institute for Health and Care Excellence (NICE) adopted the Kidney Disease: Improving Global Outcomes (KDIGO) guideline nomenclature of CKD classification, which categorises CKD and the risk of progression, using both eGFR and albuminuria levels (Figure 1)3,9. This article focuses on CKD G3a-5 and albuminuria categories 2-3, as these carry the higher risk of CKD progression3,7.
Figure 1: KDIGO CKD classification (reproduced with permission)
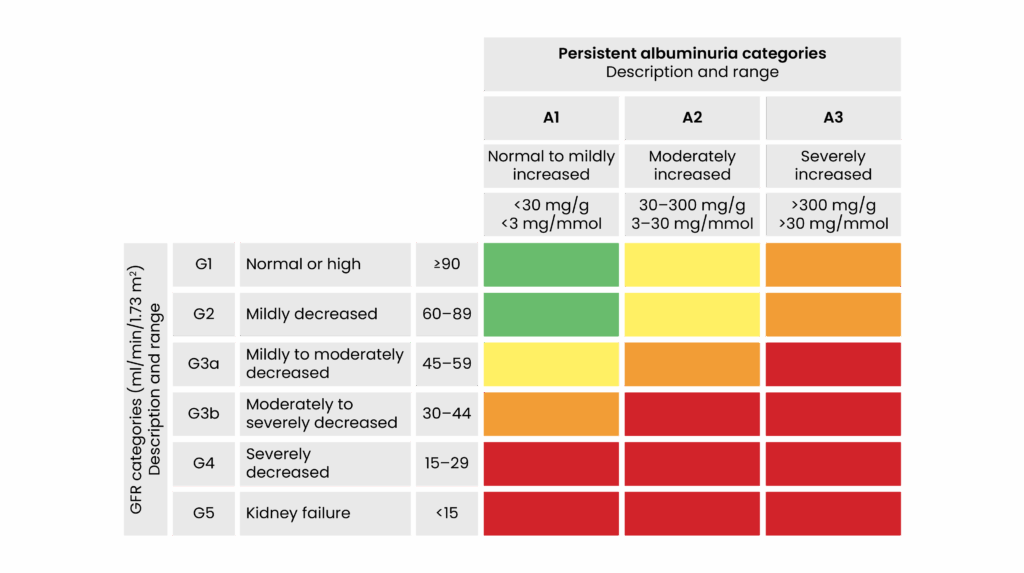
Stevens PE, Ahmed SB, Carrero JJ, et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease.
Beyond classification, tools such as the Kidney Failure Risk Equation (KFRE) estimates the two- and five-year risk of kidney failure in patients with CKD G3a-5 using age, sex, eGFR and uACR10. A score above 5% typically indicates the need for specialist renal referral10. Incorporating KFRE into routine practice enhances risk stratification and helps identify patients who would benefit most from timely intervention or specialist care.
Even modest levels of albuminuria (i.e. a uACR of 3-29 mg/mmol) or an eGFR below 60ml/min/1.73m2 are associated with nearly double the risk of CVD mortality11 compared with those within the normal reference range. Similar trends can be noted with decline in eGFR, where data suggests those with a eGFR of less than 30mL/min/1.73m2 face up to a three-times higher risk of CVD mortality, compared with those without kidney disease (see Figure 2)11. Noting the importance of eGFR and uACR, both must be considered when assessing for CVD risk stratification.
Figure 2: The individual correlations between kidney function, albumin:creatinine ratio and CV mortality
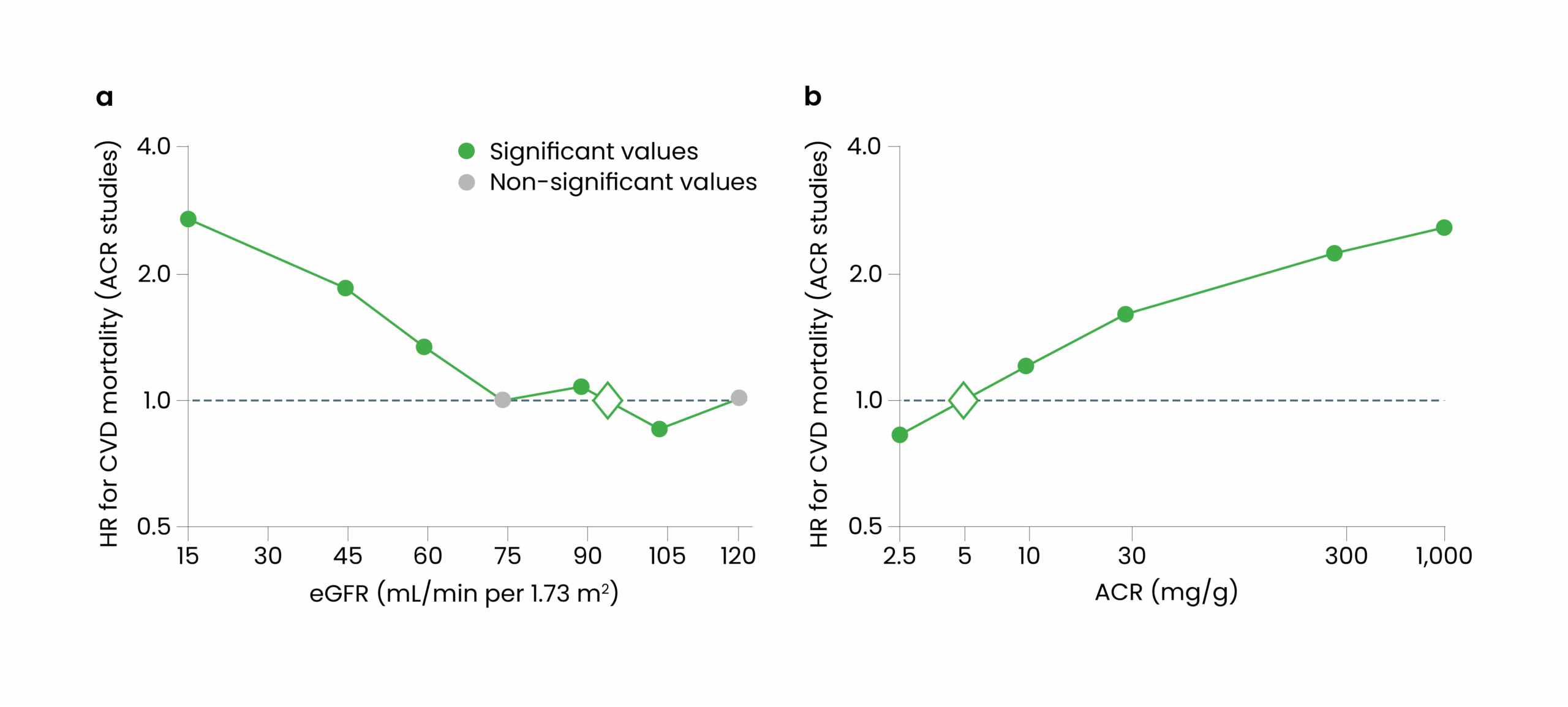
There is a 20-fold increased risk of death with CVD compared with ESKD in CKD12. In addition, for every 100 patients with CKD G3a-5, there are potentially seven acute kidney injuries (AKI), six CVD events and two intensive care unit (ICU) admissions per year13.
Prevalence and detection
According to Quality and Outcomes Framework (QOF) data, the prevalence of GP-recorded CKD G3a-5, which is defined as eGFR < 60 mL/min, including category A1-3, in England was 4.3% in 2009/201014 and 4.4% in 2023/202415, an increase of around 420,000 patients.
Prevalence data is also available through CVDPREVENT, which is a national audit of GP records in England, aimed at improving cardiovascular health in primary care16. The audit collects data on various CVD risk factors, such as CKD, hypertension, diabetes and cholesterol levels. However, unlike the QOF, CVDPREVENT includes uACR data for patients with CKD, making it a more reliable indicator of disease burden, particularly given the increased cardiovascular risk associated with albuminuria. This difference highlights an area for enhancement in the QOF, as it does not currently capture the full picture of CKD risk and progression.
Figure 3 shows the prevalence of CKD G3a-5 in each regional area of England, as of September 202417. Given the predicted increase in individuals thought to have CKD, there could be a growing number of unrecorded cases. As such, there is an opportunity for local CKD initiatives and projects to align with high-impact changes for better screening, detection and appropriate recording of individuals with CKD.
Figure 3: Proportion of prevalence (%) of GP recorded CKD (G3a-5) in regional areas in England extracted from CVDPREVENT audit data September 2024
According to CVDPREVENT data, 47% of CKD G3a-5 patients in England completed an uACR test within the past 12 months18. NICE guidance, published in 2021, recommends that the frequency of uACR and eGFR testing should be determined by CKD severity and presence of comorbid conditions9. Optimal care standards are currently falling short. For example, only 30% of patients with GP-recorded hypertension — a high-risk condition — underwent uACR testing during this period19. Several conditions are classified as high risk for CKD owing to their strong association with developing kidney damage, CKD and disease progression (e.g. hypertension, diabetes, CVD, gout, previous AKI)9,20. The UK Kidney Association (UKKA), in line with NICE, emphasises that individuals with these high-risk conditions or a family history of kidney disease should have their uACR tested at least annually to support early detection of kidney damage9,20.
In addition, uACR has been shown to be a superior indicator for CVD outcomes in people of South Asian descent, with elevated levels in this demographic being strongly associated with increased rates of CVD and cardiovascular mortality6. These findings indicate significant room for improvement in implementing recommended uACR testing practices for both CKD detection and ongoing monitoring, which also impacts CVD risk reduction.
Coding in primary care
The National Chronic Kidney Disease Audit, published in 2017, revealed significant variability in coding accuracy in England and Wales. The range of potentially uncoded cases was 0–80%, while 11% of cases were coded for CKD without biochemical evidence, particularly among the Black ethnicity group13. The audit considers several contributing factors, primarily inadequate testing among high-risk groups and inconsistencies in historical application of ethnicity-based adjustments to eGFR values, which may have affected both diagnosis and coding13. Importantly, these adjustments are no longer recommended. In 2021, NICE removed the use of ethnic correction factors from kidney function calculations, following evidence that such adjustments were based on outdated assumptions about genetic differences, rather than socio-environmental determinants of health21. Additional complexities arose from fluctuating eGFR values and insufficient availability of sequential eGFR measurements at least three months apart, both of which are essential for confirming CKD diagnosis11.
To address this, GP Systematised Nomenclature of Medicine Clinical Terms (SNOMED-CT) codes should be selected with care from the 122 codes available for CKD, using codes that follow the G staging (based on eGFR), followed by A staging (based on ACR)22. Comorbidities must be coded separately. Patients with prior kidney transplants, including donor nephrectomies, should retain CKD diagnosis code20. Furthermore, patients who have experienced AKI stage 1–3 should be reassessed for underlying CKD and coded accordingly if confirmed9. Strengthening the accuracy and consistency of coding will support better risk stratification, treatment optimisation, disease progression review and long-term patient outcomes.
Health inequalities
Minority ethnic groups face significant barriers in accessing healthcare services. This contributes to up to a five times higher rate of CKD diagnosis and progression to kidney failure, necessitating interventions such as dialysis or transplantation6. The disparity continues in treatment approaches with ethnic minority patients tending to receive dialysis instead of transplantation, despite the better clinical outcomes of the latter6.
Disparities are multifactorial and are linked with increased health inequalities, which is reflected in Dahlgren and Whitehead’s ‘Social determinants of health’ (see Figure 4)23. A report from the Health Foundation, published in 2024, emphasises the need to address these inequalities, as people in the 10% most deprived areas in England are expected to be diagnosed with major illnesses nearly a decade earlier than those in the least deprived areas — particularly in relation to diabetes, in which incidences of the condition are predicted to increase at a faster rate in the most deprived areas24. Around 40% of individuals with either type 1 or type 2 diabetes will develop CKD throughout their lifetime25. Those of Asian heritage with type 1 diabetes are around twice as likely to develop kidney failure, compared with people of white ethnicity26.
Figure 4: Dahlgren and Whitehead’s ‘Social determinants of health’
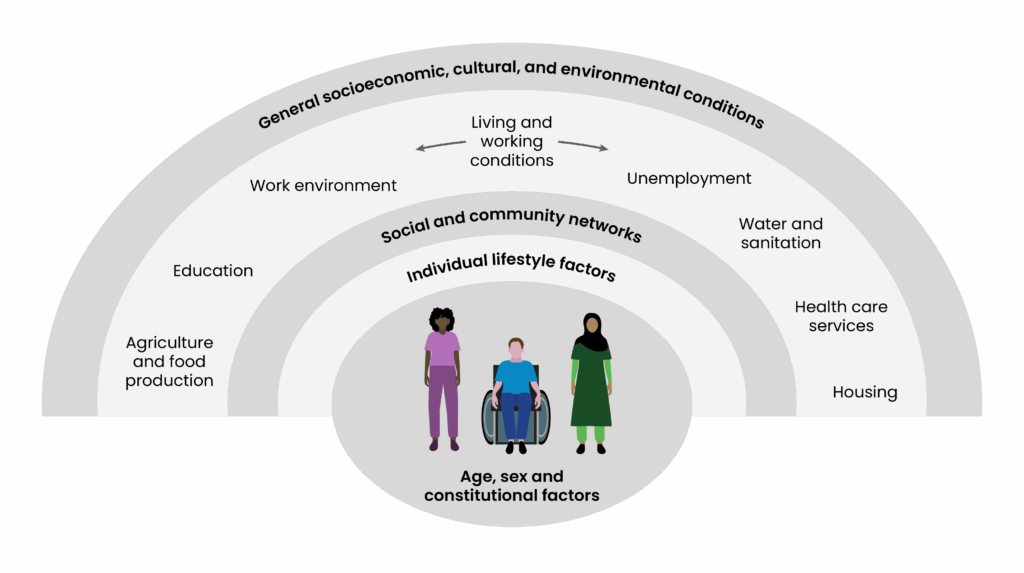
Data from the UK Renal Registry, published in 2023, show that from 2014–2020 individuals living in the most deprived areas made up the largest proportion of those starting kidney failure treatment, with 25% of patients coming from the most deprived areas of England and Wales compared with just 15% of patients from the least deprived areas27. Ethnic minority groups, particularly Black and Asian populations, are disproportionately represented in these deprived groups27. In contrast, 83% of white patients are in the least deprived group27.
These patterns reinforce that CKD is not just a medical issue but one closely linked with social and structural inequalities, disproportionately affecting those from economically disadvantaged backgrounds and ethnic minorities. This trend is also exacerbated by demographic shifts towards an ageing population, coupled with rising incidence of conditions such as diabetes, hypertension and CVD6.
NHS England data, published in 2024, show that from 2021–2022, 8% of adults aged 35–44 years and over live with CKD G1-5, which is defined as CKD G1-2 with albuminuria or CKD G3a-5 regardless of albuminuria. Of those adults living with CKD G1-5, 1% had CKD G3a-5 (See Figure 5)28. Prevalence and severity rise sharply with age. Figure 6 illustrates a stepwise increase in disease burden in individuals aged 75 years and over. A total of 48% of people in that age range have CKD G1-5, with over one-third (36%) experiencing more severe stages of CKD G3a-519. Among adults aged 35 years and over with CKD G1-5, women exhibited a higher prevalence rate of 24%, while men had a prevalence rate of 20%19. These age- and sex-based differences reflect important health inequalities, as older adults and women experience a disproportionately greater burden of CKD, yet both groups face barriers in timely diagnosis and access to optimal therapies. Early identification of all patients is crucial to narrowing health inequalities.
The CVDPREVENT audit incorporates health inequalities data. This aligns with initiatives such as Core20PLUS529, which is aimed to address health inequalities and enhance understanding and improvement of cardiovascular health in the most deprived 20% of the population (CORE20) and other vulnerable groups not captured within this cohort29.
According to CVDPREVENT data, in September 2024, there were 255,347 GP registered patients in England, aged 18 years and over, with two consecutive reduced eGFRs (<60 mL/min/1.73m2) but no recorded diagnosis of CKD G3a-530. This is referred to as ‘uncoded’ cases on GP clinical systems. Of this cohort, 38,223 (15%) belonged to the CORE20 most deprived quintile (see Figure 6)26,30. When also including patients with a latest single eGFR of <60 mL/min/1.73m2, the number of high-risk but uncoded patients rises to 579,293, with 318,931 women being disproportionately affected, compared with 260,362 men.
This gender gap in uncoded CKD is more evident in older age groups, where undiagnosed disease or unrecorded diagnosed disease accumulates. Furthermore, 96,249 of these patients are in the most deprived quintile. While, the trend across deprivation quintiles suggest CKD is present across all groups, the burden is more substantial in deprived populations31. This highlights the importance of bridging the gap in sociodemographic health inequalities and early identification of CKD, as this cumulatively accounts for an estimated 834,640 uncoded patients with CKD.
Figure 6: CKD G3a-5 prevalence rate across England by gender and deprivation quintile extracted from CVDPREVENT audit data (September 2024)
Data on social determinants of health from a study by Nelson et al., published in 2020, found that:
- Individuals with low income face a 1.5-fold increased risk of developing CKD G3a-5. Similarly, the Kidney Research UK report highlights that patients with CKD earning £12,500 annually experience twice the risk of adverse cardiovascular outcomes compared with their higher-income counterparts;
- Residing in the most deprived areas raises the risk of CKD G3a-5 by 1.55-fold;
- Individuals who rent their homes have a 1.36-fold increased risk of developing CKD G3a-5 compared with homeowners6,29,32.
Several other inequities influence kidney health outcomes, as highlighted in the Kidney Research UK’s ‘Time to act on kidney health inequalities’ report, published in 2024. For example, women are less likely to start dialysis, even though they are more frequently diagnosed with CKD. When access to care is already limited, co-existing mental health disorders can intensify the burden of CKD and are linked to poorer care outcomes. Disparities also exist in access to kidney transplantation and post-transplant outcomes among underrepresented groups, including those defined by immigration status, sexuality, gender identity, rural location and disability33.
In the UK, poor health literacy affects around 10 million adults, while one-quarter of individuals with CKD have a low level of health literacy33. This lack of understanding is associated with unhealthy lifestyles, underutilisation of preventive care and increased reliance on emergency services33. Poor health literacy leads to higher healthcare costs for the system, and it has been estimated to cost the NHS between £2.95bn and £4.92bn annually34.
Understanding these disparities is crucial to developing targeted interventions to improve the prevention and management of CKD among disadvantaged populations. To support this, healthcare providers must communicate and educate the whole CKD population through co-designing interventions in partnership with those living with CKD, particularly focusing on improving health literacy.
Treatment
CKD prevention and slowing disease progression
The current NICE guidelines for the pharmacological management of CKD, summarised in Figure 7, focus on blood pressure control, lipid-lowering therapy for primary prevention, SGLT2 inhibitors and finerenone9,35–38. Those factors have shown significant benefit in slowing CKD progression and reducing cardiovascular risk by addressing pathophysiological processes. It is important to note that the use of renin-angiotensin-aldosterone system (RAAS) inhibitors is not limited to hypertensive patients but also in patients with persistent albuminuria, regardless of blood pressure, as an independent factor to reduce albuminuria and protect renal function. This includes patients with preserved eGFR (>60mL/min/1.73m2) and A2/A3 albuminuria, who meet the criteria for CKD9.
Figure 7: Summary of NICE CKD guidelines in relation to blood pressure control, lipid lowering therapy, SGLT2i and Finerenone
NICE guidelines also emphasise individualising treatment based on patient factors, such as frailty, comorbidities, diabetes status, albuminuria levels and cardiovascular risk. It also aligns with recommendations from other major kidney disease guidelines — for example, KDIGO, ensuring consistent care approaches9,35–38.
Currently, in England, 71% of patients coded CKD G3a-5 with hypertension and proteinuria that are being treated with RAAS inhibitors39, leaving an unmet treatment need of at least 59,843 eligible patients who are not receiving therapy. RAAS inhibitors, such as angiotensin-converting enzyme (ACE) inhibitors, help lower blood pressure, reduce albuminuria and slow down the progression of kidney damage. By dilating blood vessels and reducing the workload on the kidneys, RAAS inhibitors can help preserve renal function, delay the need for dialysis or kidney transplantation and decrease the risk of CVD events40.
CVDPREVENT data demonstrates that a total of 68% of CKD G3a-5 patients in England are being treated with lipid lowering therapy (LLT)41. This data reveals that 697,448 patients are receiving suboptimal care, with the lowest LLT initiation rates being observed in the younger population who would benefit most from LLT. The SHARP trial outcomes, published in 2011, demonstrated a link between worsening kidney function and increased risk of CVD, which led to NICE recommending primary prevention of CVD for all patients with CKD. The trial estimated that for every 1,000 patients on LLT for 5 years, 30–40 major atherosclerotic events would be prevented42.
Through the CREDENCE, DAPA-CKD and EMPA-KIDNEY trials, SGLT2 inhibitors have been shown to reduce the risk of CVD, kidney dysfunction decline and death43–45. In CKD, SGLT2 inhibitors work by blocking the SGLT2 protein, leading to reduced pressure and inflammation in the kidneys, as well as stopping albuminuria and decreasing blood pressure and body weight. This novel treatment for CKD was approved by NICE in 2021, initially for patients with type 2 diabetes mellitus and, subsequently, for CKD alone, with specific prescribing criteria35. Kidney Research UK estimates that around 19% of patients with CKD are eligible for SGLT2 inhibitor therapy6.
While comprehensive guidelines for newer therapeutic options, such as SGLT2 inhibitors, are readily accessible, notable disparities persist in their adoption across the CKD patient population. A study published in The Lancet in 2024, which analysed the implementation of SGLT2 inhibitors in 738 volunteer UK-based primary care practices, revealed that out of 516,491 patients with CKD, 138,183 patients were eligible for SGLT2 inhibitors; however, only 17% (n=23,466) of patients were prescribed it46. Therefore, approximately only one in six eligible patients within this cross-sectional study received SGLT2 inhibitors for CKD in primary care.
Finerenone, a novel non-steroidal selective mineralocorticoid receptor antagonist (MRA), has emerged as another treatment option for treating CKD G3a-4 (with albuminuria) in type 2 diabetes mellitus patients38. The FIDELTY meta-analysis, published in 2021, which integrated the FIDELIO-DKD and FIGARO-DKD trials, outlined the cardiovascular and renal benefits finerenone provides in CKD47. This comprehensive analysis led to the approval of finerenone by NICE in 202338. The FIDELIO-DKD trial, with its primary focus on renal endpoints, demonstrated statistically significant results47. In contrast, the FIGARO-DKD trial centred on cardiovascular outcomes, while including renal endpoints for less-advanced CKD patients47. Notably, both trials achieved substantial benefits without a significant imbalance in adverse events, highlighting the favourable safety profile of finerenone47. Finerenone’s proven efficacy across various stages of disease progression represents a significant advancement in managing cardiorenal risk for this high-risk patient population, which also strengthens the importance of measuring urine ACR in patients with preserved kidney function and type 2 diabetes mellitus.
Innovation
Detection and treatment approaches
An important challenge lies in identifying early CKD, which can be asymptomatic6. While inexpensive and reliable dual diagnostic tests with eGFR and albuminuria can support detection, limited primary care capacity hinders routine CKD screening. This limitation can cascade into coding inconsistencies, and initial diagnoses may remain static despite disease progression. By first identifying individuals with CKD, teams can facilitate and sustain opportunities for meaningful discussions between patients and clinicians, as well as support patient education about the condition. This also enables risk stratification for clinical reviews and timely initiation or optimisation of CKD therapy, which in the long-term will reduce the progression of CVD complications, prevent ESKD and reduce costs48.
The integration of search tools within GP clinical systems represents an innovative approach to case finding, supporting healthcare providers to identify and manage patients with an array of conditions. This digital solution enables rapid risk stratification and comprehensive benchmarking of GP practices, enhancing both efficiency and quality of patient care. Several tools have emerged to support the CKD initiative. For example, the Clinical Digital Resource Collaborative (CDRC) provides population reporting searches that facilitate the prevention, identification, monitoring and management of both coded and un-coded CKD cases49. Similarly, the APL-Renal tool, created by the Clinical Effectiveness Group (CEG), Queen Mary University London (QMUL), is being used in north east London (NEL) to capture vital data such as eGFR trends, urine ACR values, comorbidities, blood pressure and prescriptions, such as RAAS inhibitors, SGLT2 inhibitors and statins50.
The effective utilisation of these tools requires a strategic shift in healthcare delivery. The search tool embedment alone is insufficient; however, the answer lies in resource allocation for data analysis. Rather than pursuing individual GP practice-level solutions, which would create inefficiencies and inconsistencies, a more effective approach involves system-level coordination. This can be achieved through various channels, such as through primary care coordination via primary care network (PCN) pharmacist collaboration or integrated care board (ICB) pharmacist integration, in addition to having specialist support, such as a secondary care pharmacist-led multidisciplinary team, assisting with the provision of data insights effectively, while ensuring comprehensive and tailored care delivery while maintaining operational efficiency across the healthcare systems. In the NEL CKD programme, participating GP practices underwent a structured onboarding, including a pre-programme survey and baseline needs assessment and a virtual introductory webinar introducing the programme. The session covered CKD coding, use of APL-Renal CEG tools and templates, and alignment with NICE and London Kidney Network guidance.
A revolutionary approach to healthcare delivery is through Healthy.io’s Minuteful Kidney service, which transforms smartphones into clinical-grade medical devices for at-home urine self-testing for high-risk patients and those with diabetes who have never had a uACR test done51. Instead of visiting a clinic, patients receive a kit by post, perform the test themselves at home — guided by a smartphone app — and send digital results to their GP for review51. This innovative platform leverages advanced technology to bridge the gap between healthcare providers and patients, making medical testing more accessible and convenient. The service’s impact across 16 ICBs has been remarkable, with over 74,000 patients successfully completing their tests and leading to the detection of more than 8,500 additional CKD cases52 .
These achievements have translated into significant projected healthcare improvements, including annual reductions of 595 cases of AKI, 510 CVD events, 95 ICU admissions and 3,230 unplanned hospital admissions52. The platform’s accessibility benefits extend to traditionally underserved populations, including housebound patients, those with limited mobility and individuals with scheduling constraints, such as carers and working professionals52. This approach has proven highly effective, increasing test completion rates by up to 50% among previously unengaged patient populations52. However, two crucial challenges temper these successes. The solution’s effectiveness is partially limited by its reliance on smartphone access, highlighting ongoing challenges in fully addressing healthcare inequalities through digital transformation52. Additionally, the implementation of home uACR testing services relies at the discretion of individual GP practices.
There are comprehensive networks and pilot programmes across the UK to guide and implement recommendations from comprehensively reviewed and benchmarked data from the ‘Getting It Right the First Time’ national report, published in 2024, and the ‘Renal service transformation programme’, which engage with various stakeholders, such as ICB’s, charities, such as Kidney Care UK, and NHS England regional teams to review specialities and implement evidence-based recommendations53,54.
At local and regional levels, there are projects which have strategically prioritised CKD and guideline directed medical therapy in practice (See Table 1)55–57.
Table 1: Examples of local projects aiming to improve medical therapy of CKD
By standardising care pathways and promoting proactive CKD management, these initiatives are aimed to improve outcomes, through digital transformation, shared decision-making and personalised follow-up models, to reduce disease progression and enhance equity in treatment access.
Treatment optimisation
An audit conducted by East and North Hertfordshire NHS Trust in 2024 highlighted disparities in how crucial medications for CKD are prescribed via RAG (red, amber, green) ratings in ICB formularies across England. Between May and June 2024, 88% of ICBs approved dapagliflozin for primary care initiation, and 83% of ICBs approved empagliflozin for primary care initiation. However, finerenone fared poorly, with only 22% of ICBs assigning it a green rating58. 42% of ICBs rated finerenone amber, while 22% deemed it red, effectively restricting its use to secondary or tertiary care settings58. This stark variation with finerenone may be owing to it being a new medication with limited familiarity, requiring more training, commissioning decisions by ICBs, concerns about cost and the requirement for close monitoring because of risks such as hyperkalaemia. Having a green rating enables timely access to medicines in primary care settings, allowing patients to receive medications that enhance CKD progression control, without delay. The RAG system for CKD therapies potentially contributes and widens the inequitable access to transformative treatments, as it effectively creates a two-tier system where available treatment depends on geographical locations. With the emergence of newer treatments for CKD, such as finerenone38 and GLP-1 receptor agonists59, which are not yet approved, it highlights the need for more standardised, collaborative health system approaches and quality indicators to improve equity of access to treatment management. This presents opportunities for developing more efficient care coordination systems, to provide more consistent access to crucial CKD treatments.
While national data on SGLT2 inhibitor prescriptions exist through OpenPrescribing, there is currently no specific national database for the number of patients prescribed SGLT2 inhibitors for CKD60. There is a growing recognition of the benefits of SGLT2 inhibitors for CKD, shifting focus from traditional endpoints, such as blood sugar control, to kidney-specific outcomes, such as changes in eGFR and albuminuria levels, incidence of ESKD, prognosis and mortality61. Establishing a benchmark for the current status and usage of SGLT2 inhibitors for CKD will, therefore, allow for a gap analysis review and evaluate areas where improvements can be made.
The importance of such data is demonstrated by existing ‘Size of the prize’ data for CVD, as it quantifies potential benefits and guides resource allocation62. It allows policymakers to prioritise evidence-based interventions, conduct cost-benefit analyses, set realistic goals and monitor progress, influence funding decisions and compare different approaches to maximise public health impact. For example, UCLPartners ‘Size of the Prize’ data shows that optimising treatment for 80% of patients with high blood pressure would prevent 17,000 strokes and heart attacks over three years, saving £200m for the NHS62. Similarly, treating 90% of CVD patients with lipid lowering therapy would prevent around 9,000 strokes and heart attacks over three years62. If treatment coverage reached 95%, this would prevent over 18,000 CVD events, saving the NHS £1.39m per hundred stroke patients and £746,600 per hundred heart attack patients62.
Health inequality
The effective management of CKD requires a collaborative approach between healthcare professionals, policymakers and stakeholders. While improvements in healthcare delivery systems and CKD management are essential for reducing missed opportunities in CKD identification and progression, it is also important to empower patients in managing their health outcomes. CKD is a public health crisis and requires a unified response. Understanding how various aspects of an individual’s identity and circumstances intersect to shape their health experiences and interactions with healthcare systems is important in developing more effective strategies to address health inequalities and improve outcomes for diverse patient populations. This is called intersectionality and aligns with Dahlgren and Whitehead’s ‘Wider social determinants of health’23.
This presents opportunities for improvement. Modifiable risk factors, such as diet, represent significant challenges that can impact not only patients living with CKD, but also their families and wider communities. Often it is an issue of time constraints, pester power from children or family members, limited knowledge on how to cook healthy and cost-effective meals, or making cost-effective shopping decisions. The ongoing cost-of-living crisis has exacerbated these existing healthcare challenges, highlighting the need for innovative solutions. To address these pressures, healthcare providers have and should continue to engage in collaborative approaches that bring together various stakeholders to support patient care. Collective efforts create valuable spaces where patients can engage in informed discussions and make empowered decisions about their health, fostering a more supportive and inclusive healthcare environment. For example:
- The Scottish peer educator programme collaborates with faith-based groups in the South Asian community to provide education and resources on kidney health, relevant policies and treatments5;
- Group face-to-face consultations, an innovative concept for primary care settings, where multiple patients with similar conditions meet together with healthcare professionals. This collaborative model has shown significant benefits in chronic disease management63,64 and, therefore, may be useful in conditions such CKD, diabetes and CVD. Not only does it provide space to discuss clinical management of the condition but also the psychological and social aspects with managing a long-term condition64;
- Healthcare professionals play a vital role in connecting patients with valuable resources such as Kidney Care UK’s ‘Kidney Kitchen’ and ‘Bloody Amazing Kidneys’ campaign, which aim to educate patients about CKD risk factors, lifestyle modifications, encouraging testing among high-risk groups and to engage with routine monitoring and follow-up appointments more seriously65,66. Similarly, there are factsheets available for patients by ‘plant-based health professionals’ in relation to diet and exercise for a variety of conditions, including CKD67.
By fostering collaborative relationships between healthcare organisations, charities and local communities, we can empower patients and facilitate informed decision-making6. Such approaches appear to provide benefit to individual patients but also contribute to reducing the overall population health burden related to CKD and its associated cardiovascular risks.
Conclusion
Approaches to CKD management currently vary across regions. In response to the growing demands of the condition, numerous ongoing initiatives, frameworks and programmes are being implemented to improve CKD strategies. These proactive efforts demonstrate a growing recognition of the need for more coordinated care approaches, particularly in addressing the increasing burden of CKD and its associated cardiovascular risks.
The landscape of CKD management in the UK is evolving. There are ongoing medication trials, new treatment approaches and comprehensive care programmes. These initiatives span the entire spectrum of CKD care, from early detection and diagnosis to long-term management, with a focus on ensuring equitable access to care for all patients. There is also a strong emphasis on education and training for both clinicians and patients, aiming to create a more coordinated progressive approach to managing CKD.
As we stand at the threshold of a CKD epidemic, the urgency for action is critical. Without consistent and integrative measures, we risk overwhelming our healthcare systems and increasing the likelihood of premature dialysis, CVD and death. However, this challenge also presents an opportunity to transform the trajectory of CKD management in the UK through successful implementation of comprehensive, equitable and multifaceted strategies to delay the progression of CKD.
By integrating best practices and implementing evidence-based guidelines, while addressing systemic inequalities in access to care, particularly within ethnic minority groups and socio-economically underserved groups, we can dramatically reduce kidney and cardiovascular risk across the population. Public engagement is paramount in achieving these goals. Through collective efforts, a sustainable healthcare system that prevents, detects and manages this disease with unprecedented effectiveness can be achieved.
- 1.Francis A, Harhay MN, Ong ACM, et al. Chronic kidney disease and the global public health agenda: an international consensus. Nat Rev Nephrol. 2024;20(7):473-485. doi:10.1038/s41581-024-00820-6
- 2.Reducing the burden of noncommunicable diseases through promotion of kidney health and strengthening prevention and control of kidney disease. World Health Organization . 2025. https://apps.who.int/gb/ebwha/pdf_files/EB156/B156_CONF6-en.pdf
- 3.Stevens PE, Ahmed SB, Carrero JJ, et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Nat Rev Nephrol. 2024;105(4):S117-S314. doi:10.1016/j.kint.2023.10.018
- 4.Matsushita K, Ballew SH, Wang AYM, Kalyesubula R. Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. European Journal of General Practice. 2022;18(11):696-707. doi:10.1038/s41581-022-00616-6
- 5.Kendir C, van den Akker M, Vos R, Metsemakers J. Cardiovascular disease patients have increased risk for comorbidity: A cross-sectional study in the Netherlands. European Journal of General Practice. November 23, 2017. http://dx.doi.org/10.1080/13814788.2017.1398318
- 6.Kidney disease: A UK public health emergency: the health economics of kidney disease to 2033. Kidney International. Published online 2023. https://www.kidneyresearchuk.org/wp-content/uploads/2023/06/Economics-of-Kidney-Disease-full-report_accessible.pdf
- 7.Butt L, Unnersjö-Jess D, Höhne M, et al. A molecular mechanism explaining albuminuria in kidney disease. Nat Metab. 2020;2(5):461-474. doi:10.1038/s42255-020-0204-y
- 8.K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. National Kidney Foundation. 2002. https://pubmed.ncbi.nlm.nih.gov/11904577/
- 9.Chronic kidney disease: assessment and management. The National Institute for Health and Care Excellence . 2021. https://www.nice.org.uk/guidance/ng203/chapter/Recommendations
- 10.The Kidney Failure Risk Equation UK. The Kidney Failure Risk Equation UK. https://www.kidneyfailurerisk.co.uk/
- 11.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. The Lancet. 2013;382(9889):339-352. doi:10.1016/s0140-6736(13)60595-4
- 12.CVD prevention: chronic kidney disease detection and management. The National Institute for Health and Care Excellence. https://stpsupport.nice.org.uk/cvd-prevention-ckd/index.html
- 13.Nitsch D, Caplin B, Hull S, Wheeler D. National Chronic Kidney Disease Audit. The London School of Hygiene & Tropical Medicine. 2017. https://www.lshtm.ac.uk/files/ckd_audit_report.pdf
- 14.Kerr M. Chronic Kidney Disease in England: The Human and Financial Cost. NHS England . 2017. https://www.england.nhs.uk/improvement-hub/wp-content/uploads/sites/44/2017/11/Chronic-Kidney-Disease-in-England-The-Human-and-Financial-Cost.pdf
- 15.Quality and Outcomes Framework 2023-24: Prevalence, achievement and personalised care adjustments – National and Region level. NHS England . 2024. https://digital.nhs.uk/data-and-information/publications/statistical/quality-and-outcomes-framework-achievement-prevalence-and-exceptions-data/2023-24
- 16.CVDPREVENT. CVDPREVENT. https://www.cvdprevent.nhs.uk/about
- 17.Prevalence of GP recorded CKD (G3a to G5) . CVDPREVENT. 2025. https://data.cvdprevent.nhs.uk/data-explorer?period=20&level=1&area=1&indicator=8
- 18.Patients with GP recorded CKD (G3a to G5) with a record of a urine ACR test in the preceding 12 months. CVDPREVENT. 2025. https://data.cvdprevent.nhs.uk/data-explorer?period=20&level=1&area=1&indicator=17
- 19.Patients with GP recorded hypertension, with a record of a urine ACR test in the preceding 12 months . CVDPREVENT. 2025. https://data.cvdprevent.nhs.uk/data-explorer?period=20&level=1&area=1&indicator=52
- 20.The UK eCKD Guide. UK Kidney Association. 2024. https://www.ukkidney.org/health-professionals/information-resources/uk-eckd-guide
- 21.The rationale for removing adjustment for ethnicity from eGFRcreatinine and recommendations for implementation of the change in practice. UK Kidney Association. 2021. https://www.ukkidney.org/sites/renal.org/files/guidelines/Rationale_and_recommendations_for_implementation_NICE%20CKD%20Guideline%202021.pdf
- 22.Guidelines on Chronic Kidney Disease Coding in Primary Care London Kidney Network Expert Consensus Background. London Kidney Network. 2024. https://londonkidneynetwork.nhs.uk/wp-content/uploads/2024/07/LKN-CKD-Coding-Guidelines-27.6.24-final-v2.2.pdf
- 23.Social Determinants of Health. Public Health England. 2017. https://www.gov.uk/government/publications/health-profile-for-england/chapter-6-social-determinants-of-health
- 24.Raymond A, Wyatt T, Douglas H, Head A, Kypridemos C, Rachet-Jacquet L. Health inequalities in 2040. The Health Foundation. 2024. https://www.health.org.uk/reports-and-analysis/reports/health-inequalities-in-2040?gad_source=1&gclid=Cj0KCQiAr7C6BhDRARIsAOUKifhVLYixy6QsKTBSyJGoEjKWq8XC-u6_SBIjAw62woRpSlEqrmtN7vwaAiUSEALw_wcB
- 25.Shared professional practice on kidney care and diabetes. Diabetes UK. https://www.diabetes.org.uk/for-professionals/improving-care/good-practice/kidney-care
- 26.Kidney disease in people from minority ethnic groups. Kidney Research UK. https://www.kidneyresearchuk.org/kidney-health-information/about-kidney-disease/am-i-at-risk/kidney-disease-in-minority-ethnic-groups/
- 27.Social and economic disparities in patients with kidney failure in England and Wales. Kidney Research UK. 2023. https://www.ukkidney.org/sites/default/files/Socioeconomic%20Disparities%202023%20Final.pdf
- 28.Health survey for England, 2022 part 2. NHS England. 2024. https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/2022-part-2
- 29.Core20PLUS5 an Approach to Reducing Health Inequalities. NHS England. 2021. https://www.england.nhs.uk/about/equality/equality-hub/national-healthcare-inequalities-improvement-programme/core20plus5/
- 30.Patients whose last two eGFRs are less than 60ml/min/1.73m2 (uncoded CKD), who do not have a record of GP recorded CKD (G3a to G5). CVDPREVENT. 2025. https://data.cvdprevent.nhs.uk/data-explorer?period=20&level=1&area=1&indicator=13
- 31.Patients whose last single eGFR is less than 60ml/min/1.73m2 (at risk of CKD), who do not have a record of GP recorded CKD (G3a to G5). CVDPREVENT. 2025. https://data.cvdprevent.nhs.uk/data-explorer?period=20&level=1&area=1&indicator=15
- 32.Nelson ML, Buchanan-Peart KAR, Oribhabor GI, Khokale RV, Cancarevic I. Survival of the Fittest: Addressing the Disparities in the Burden of Chronic Kidney Disease. Cureus. Published online July 31, 2020. doi:10.7759/cureus.9499
- 33.Time To Act: A New Review of Kidney Health Inequalities. Kidney Research UK. 2024. https://www.kidneyresearchuk.org/wp-content/uploads/2024/07/FINAL-Accessible-full-report-_Academic_Report_V7-23R02.pdf
- 34.Local action on health inequalities Improving health literacy to reduce health inequalities. Public Health England. 2015. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/460709/4a_Health_Literacy-Full.pdf
- 35.Cardiovascular disease: risk assessment and reduction, including lipid modification. The National Institute for Health and Care Excellence. 2023. https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
- 36.Dapagliflozin for treating chronic kidney disease. The National Institute for Health and Care Excellence. 2025. https://www.nice.org.uk/guidance/ta1075/chapter/1-Recommendations
- 37.Empagliflozin for treating chronic kidney disease. The National Institute for Health and Care Excellence. 2023. https://www.nice.org.uk/guidance/TA942/chapter/1-Recommendations
- 38.Finerenone for treating chronic kidney disease in type 2 diabetes. The National Institute for Health and Care Excellence. 2023. https://www.nice.org.uk/guidance/ta877/chapter/1-Recommendations
- 39.Patients with GP recorded CKD (G3a to G5) and hypertension and proteinuria, who are currently treated with renin-angiotensin system antagonists. CVDPREVENT. 2025. https://data.cvdprevent.nhs.uk/data-explorer?period=20&level=1&area=1&indicator=19
- 40.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of Losartan on Renal and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Nephropathy. N Engl J Med. 2001;345(12):861-869. doi:10.1056/nejmoa011161
- 41.Patients with GP recorded chronic kidney disease (G3a to G5), who are currently treated with lipid lowering therapy. CVDPREVENT. 2025. https://data.cvdprevent.nhs.uk/data-explorer?period=20&level=1&area=1&indicator=23
- 42.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. The Lancet. 2011;377(9784):2181-2192. doi:10.1016/s0140-6736(11)60739-3
- 43.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380(24):2295-2306. doi:10.1056/nejmoa1811744
- 44.Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383(15):1436-1446. doi:10.1056/nejmoa2024816
- 45.Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2023;388(2):117-127. doi:10.1056/nejmoa2204233
- 46.Forbes AK, Hinton W, Feher MD, et al. Implementation of chronic kidney disease guidelines for sodium-glucose co-transporter-2 inhibitor use in primary care in the UK: a cross-sectional study. eClinicalMedicine. 2024;68:102426. doi:10.1016/j.eclinm.2024.102426
- 47.Agarwal R, Filippatos G, Pitt B, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. European Heart Journal. 2021;43(6):474-484. doi:10.1093/eurheartj/ehab777
- 48.Asgari E, Basudev N, Bramham K, Burke S, Carvalho C. CKD in Primary Care: new approaches to reduce inequalities and save lives LKN CKD Early Identification and Optimisation Pathways (3 in 3). London Kidney Network. 2024. https://londonkidneynetwork.nhs.uk/wp-content/uploads/2024/09/LKN-CKD-Early-Identification-Pathway-19.9.24-final-v2.3.pdf
- 49.EMIS: Acute Kidney Injury (AKI) & Chronic Kidney Disease (CKD). Clinical Digital Resource Collaborative. 2024. https://cdrc.nhs.uk/resources/emis-resource-centre/emis-specialties/emis-renal-overview/chronic-kidney-disease-ckd-emis/
- 50.APL-Renal tool User guide for EMIS. Clinical Effectiveness Group (CEG) Queen Mary University of London. 2023. https://www.qmul.ac.uk/ceg/media/ceg/documents/APL-Renal-USER-GUIDE—EMIS.pdf
- 51.Improve Adherence with Home Kidney Tests. Minuteful Kidney. https://healthy.io/services/kidney/
- 52.At-home urine self-testing using a smartphone. NHS Transformation Directorate. https://transform.england.nhs.uk/key-tools-and-info/digital-playbooks/renal-digital-playbook/at-home-urine-self-testing-using-a-smartphone/
- 53.Lipkin G, Mckane W. Renal Medicine GIRFT Programme: National Specialty Report. Getting It Right The First Time . 2021. https://gettingitrightfirsttime.co.uk/wp-content/uploads/2021/09/Renal-Medicine-Sept21k.pdf
- 54.Sinha S. Renal Service Transformation Programme . NHS England . https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/30040/renal-services-transformation-programme-s-sinha.pdf
- 55.Chronic kidney disease . Health Innovation NENC. 2024. https://healthinnovationnenc.org.uk/what-we-do/improving-population-health/cardiovascular-disease-prevention/chronic-kidney-disease/
- 56.Leicester, Leicestershire and Rutland chronic kidney disease integrated care delivery programme. Health Innovation East Midland. 2021. https://healthinnovation-em.org.uk/our-work/innovations/leicester-leicestershire-and-rutland-chronic-kidney-disease-integrated-care-delivery-programme
- 57.London Kidney Network. London Kidney Network. 2023. https://londonkidneynetwork.nhs.uk/about-us/
- 58.Morlidge C, Mallindine C. An audit of the RAG rating of SGLT2 and MRA’s for Integrated Care Boards within England . UK Kidney Association . 2024. https://www.ukkidney.org/sites/default/files/documents/RAG%20poster%202024.pdf
- 59.GLP-1 Receptor Agonists (GLP-1 RAs). National Kidney Foundation. 2024. https://www.kidney.org/kidney-topics/glp-1-receptor-agonists-glp-1-ras
- 60.Openprescribing. Openprescribing. https://openprescribing.net
- 61.Roddick AJ, Wonnacott A, Webb D, et al. UK Kidney Association Clinical Practice Guideline: Sodium-Glucose Co-transporter-2 (SGLT-2) Inhibition in Adults with Kidney Disease 2023 UPDATE. BMC Nephrol. 2023;24(1). doi:10.1186/s12882-023-03339-3
- 62.Size of the Prize for high blood pressure. UCLPartners. https://uclpartners.com/project/size-of-the-prize-for-preventing-heart-attacks-and-strokes-at-scale/
- 63.Group Consultations – Shared Medical Appointments . Wessex LMCs. 2023. https://www.wessexlmcs.com/guidance/group-consultations-shared-medical-appointments/
- 64.Group consultations: Together, patients are stronger. NHS England . https://www.england.nhs.uk/gp/case-studies/group-consultations-together-patients-are-stronger/
- 66.Celebrating our Bloody Amazing Kidneys with our new awareness campaign. Kidney Care UK. 2024. https://kidneycareuk.org/news-from-kidney-care-uk/bloodyamazingkidneys/
- 67.Factsheets. Plant Based Health Professionals UK. https://plantbasedhealthprofessionals.com/factsheets
1 comment
You must be logged in to post a comment.


Interesting article on CKD! Intersectional approaches are vital for addressing inequalities in healthcare and health systems - the article puts it well when it says "it is also important to empower patients in managing their health outcomes. CKD is a public health crisis and requires a unified response."