Abstract:
Introduction: ST-elevation myocardial infarction (STEMI) occurs as a result of plaque rupture, leading to interruption of arterial blood flow and subsequent myocardial necrosis. The incidence of hospital admission relating to STEMI in the UK is around 5 per 1000 people each year. The speed of access to reperfusion therapy in patients with STEMI cases can improve prognosis in patients. This article provides an overview of the pharmacotherapy of STEMI, with reference to recommendations from four international clinical guidelines — United States, UK, Europe, and Australia and New Zealand. The pharmacotherapy during the immediate acute period, the acute period post-reperfusion and in the secondary prevention period are discussed in this context.
Keywords: guidelines, pharmacotherapy, ST-elevation myocardial infarction, STEMI.
Original submitted: 18 July 2019; Revised submitted: 02 March 2020; Accepted for publication: 31 March 2020; Published online: 02 June 2021.
Key points
- Reperfusion therapy, either primary percutaneous coronary intervention (PCI; surgical), coronary artery bypass graft (surgical) or fibrinolytic therapy (medical) is used to restore blood flow to blocked arteries after myocardial infarction;
- Dual antiplatelet and a parenteral anticoagulant therapy are indicated for patients undergoing primary PCI or fibrinolytic therapy;
- In patients undergoing primary PCI, a glycoprotein IIb/IIIa (e.g. tirofiban) is also indicated;
- The main goal of long-term maintenance pharmacotherapy post-STEMI is to prolong survival through risk factor modification and to prevent further acute coronary episodes;
- Long-term maintenance pharmacotherapy involves aspirin, beta-blocker, lipid-modifying therapy, renin-angiotensin system inhibitor and P2Y12 inhibitor.
Introduction
Acute coronary syndrome is a term that encompasses three clinical categories of coronary artery diseases (CAD): ST-elevation myocardial infarction (STEMI); non-ST-elevation myocardial infarction (NSTEMI); and unstable angina (UA)[1]. Terminologies used to describe conditions involving the heart and blood vessels, such as ‘cardiovascular disease’, are often used interchangeably by healthcare professionals, but there are technical differences between them. Cardiovascular disease includes CAD and acute coronary syndrome (ACS), among others. CAD is characterised by atherosclerosis in the coronary arteries and can be asymptomatic, whereas ACS presents acutely and is almost always accompanied by symptoms (e.g. chest pain or discomfort, shortness of breath, sweating), and frequently associated with myocardial infarction (MI), regardless of the presence of CAD[2].
STEMI typically occurs after abrupt and catastrophic disruption of an atherosclerotic plaque, which results in the activation of platelet aggregation, thrombin generation and thrombus formation, causing interruption of blood flow[3]. If the occlusion is severe and persistent, myocardial cell necrosis follows[3]. The speed of access to effective STEMI treatment is therefore vital, as nearly half of the potentially salvageable myocardium is lost within one hour of the coronary artery being occluded, and two-thirds lost within three hours[4].
Epidemiology
The incidence of STEMI in the UK and other developed countries has been declining over the past 20 years, owing to improved health systems and public health strategies[4,5]. The incidence of STEMI varies between regions in the UK and averages around 500 hospitalised episodes per 1 million people each year[4]. In the UK, around 640,000 men and 275,000 women will have MI at some point in their lives, with 175,000 cases occurring annually[6].
In the United States, the overall prevalence of MI is around 2.8% in adults aged 20 years or over, and the estimated incidence is 550,000 new and 200,000 recurrent MIs annually[7]. Comparatively, in the UK, the overall prevalence of MI is around 2.46% in men and 0.87% in women[6]. Around 38% of patients in the United States with ACS have STEMI, compared with 47% of people with ACS in Europe[7].
MI affects both men and women, but tends to occur at a younger age in men, with the incidence increasing in women after the menopause[7]. The average age of a person having a first MI is 65.3 years for men and 71.8 years for women in the United States[7].
Prognosis
Over the past 30 years, in-hospital mortality after ACS in the UK has fallen from around 20% to around 5%, which can be attributed to factors such as access to early reperfusion and improved drug therapy[4,8]. However, around 50% of people who die of an MI do so before reaching hospital[9].
Myocardial necrosis is an important indicator of prognosis, where greater degrees of necrosis are associated with a worse prognosis[10]. Other indicators include elevated troponin levels and major bleeding, such as intracranial bleeding or fatal bleeding[11,12]. In women with STEMI, younger age is associated with higher 30-day mortality rates, even after adjustment for medicines, primary percutaneous coronary intervention and other coexisting comorbidities[13].
Adherence to evidence-based medicine has been shown to improve patient outcomes in relation to mortality and re-infarction rates[8]. The main goals of treatment in STEMI aim to limit myocardial damage by restoring myocardial blood flow as quickly as possible, and to decrease subsequent remodelling, which can have negative impacts on ventricular function and prognosis[14].
This article discusses the pharmacotherapy for STEMI, from symptom onset to secondary prevention treatment. Recommendations from four major clinical guidelines will be discussed:
- The American College of Cardiology/American Heart Association 2013 guideline;
- The National Institute for Health and Care Excellence 2013 guideline;
- The European Society of Cardiology 2017 guideline;
- The National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand 2016 guideline[4,5,15,16].
The guidelines are summarised in this article to provide a global perspective of the standards of pharmacotherapy for STEMI; however, the focus will be on UK-based management.
Sources and selection criteria
A guideline search was conducted from the earliest record until December 2020 on guideline repositories, including the Guidelines International Network and National Collaborating Centre for Chronic Conditions. Eligible guidelines focused on pharmacotherapy of ST-elevated MI. To allow for appropriate comparison, guidelines from countries of a similar income status and demographic characteristics to the UK were selected. Guidelines not published in English were ineligible. Search terms included ‘myocardial infarction’, ‘acute coronary syndrome’ and ‘guidelines’. The inclusion criteria were:
- The clinical practice guideline was produced under the support of a health professional association or society, public organisation, healthcare organisation or government agency;
- The clinical practice guideline was from a country that is a member of the G12;
- The clinical practice guideline contains systematically developed statements that include recommendations, strategies or information to guide decisions about appropriate healthcare;
- It must refer to the pharmacological management of ST-elevated myocardial infarction, in both the acute phase and secondary prevention period;
- It was published in English, from 1 January 2006 onwards.
Pharmacotherapy upon presentation of chest pain symptoms
Initial treatment for all patients presenting with symptoms of STEMI is to provide pain relief, anxiety relief and vasodilation. A single loading dose of aspirin (e.g. 300mg) should be administered as soon as possible upon presentation of ACS. Immediate pain relief may be achieved with either glyceryl trinitrate (GTN) given sublingually or intravenously, or intravenous opioids, such as morphine or diamorphine[4,5,16].
For symptom relief and regression of ST depression, GTN administered intravenously is more effective than sublingually; however, in practice, sublingual GTN is usually given first line, with intravenous GTN given if pain relief is not achieved after administration of sublingual GTN[16,17] .
An anti-emetic (commonly metoclopramide) can be given intravenously alongside parenteral opioid administration for the relief of opioid-induced nausea and vomiting. However, evidence suggests morphine use may slow the gastrointestinal absorption, and delay the onset of action, of oral antiplatelet agents (e.g. clopidogrel, ticagrelor and prasugrel)[18].
Diagnosis of ST-elevation myocardial infarction
ST-elevation usually indicates complete blockage of a coronary artery and, in most cases, warrants emergency treatment to re-open the artery, which is carried out through an appropriate reperfusion strategy[4,19].
Reperfusion therapy
There are two main types of reperfusion therapy that are used to restore blood flow to blocked arteries after STEMI: primary percutaneous coronary intervention (PCI) or fibrinolytic therapy.
In the 1980s and 1990s, fibrinolysis was the best method to restore blood flow[4]. However, this method failed to reperfuse around 20–30% of patients and caused haemorrhagic stroke in around 1% of patients[4]. Attention soon turned to primary PCI — an umbrella term that encompassed coronary angioplasty and stenting — which is both feasible and cost effective, and is now the treatment of choice for STEMI, provided it can be delivered in a timely fashion[4].
Primary PCI
Primary PCI is superior to fibrinolysis in reducing mortality, re-infarction and stroke[20]. There is no current trial data that examines the extent to which the PCI-related time delay diminishes its advantages over fibrinolysis. European and UK guidelines recommend that the absolute time from STEMI diagnosis to PCI-mediated reperfusion should be up to 120 minutes[4,15]. The US guideline recommends 90–120 minutes depending on the capacity of the centre in which the patient first presents, while the Australia and New Zealand guideline recommend within 90 minutes of first medical contact[5,16].
PCI has been used over two decades and has evolved from balloon angioplasty to bare metal stent implantation, drug-eluting stents and, more recently, biodegradable polymer stents to reduce the risk of stent thrombosis[21]. PCI has now become common practice and more than 300,000 patients undergo PCI procedures in the United States each year[22]. In the UK, a national audit estimated that primary PCI represented about 27% of all PCI activity, equivalent to a rate between 300–500 per 1 million population in each region[19]. It also found that most PCI centres in the UK achieved satisfactory ‘door-to-balloon’ times for the emergency treatment of STEMI, with 91% of patients treated within 90 minutes[19].
Fibrinolytic therapy
Fibrinolysis is an alternative to primary PCI, particularly if PCI cannot be carried out within the timeframe as outlined above. Fibrinolytic agents prevent 30 early deaths per 1000 patients treated within six hours after symptom onset[23]. The largest absolute benefit of fibrinolysis is when treatment is offered within two hours of symptom onset[24]. The later the patient presents (particularly after three hours), the more consideration should be given to transfer the patient for primary PCI as the efficacy and clinical benefit of fibrinolysis decreases as the time from symptom onset increases[25]. Fibrinolytic therapy is associated with a small but significant increase in strokes, largely owing to cerebral haemorrhage[23]. The use of fibrinolytic therapy is therefore contraindicated in patients with the following conditions:
- Uncontrolled systolic blood pressure (>180/110mmHg);
- Previous stroke/transient ischaemic attack (TIA);
- Prior intracranial haemorrhage;
- Current anticoagulation use;
- Recent trauma/surgery;
- Gastrointestinal bleeding in the past four weeks[16].
In this cohort of patients, the use of primary PCI may be preferred to fibrinolysis regardless of ‘time to reperfusion’[16].
The fibrinolytics that are available and used in the UK are tenecteplase, alteplase and streptokinase. Tenecteplase and alteplase are the most used fibrinolytics, while streptokinase use in the UK is rare, owing to the superior survival benefit of tenecteplase and alteplase[26]. Tenecteplase has a stronger fibrin specificity compared to alteplase[5]. Streptokinase and alteplase have short half-lives and must be administered by intravenous infusion[27]. Tenecteplase has a longer half-life and can be administered as a bolus injection[27]. Fibrinolysis therapy, in the same way as primary PCI, is usually co-administered with adjunct pharmacotherapies, such as aspirin, clopidogrel and anticoagulants[28].
Acute management of ST-elevation myocardial infarction
Pharmacotherapy for primary PCI
Patients undergoing primary PCI should receive dual anti-platelet therapy (DAPT) and a parenteral anticoagulant. DAPT is a combination of aspirin and a P2Y12 inhibitor, with choice of P2Y12 inhibitor and duration of DAPT influencing the balance of clinical efficacy of PCI and bleeding rates from DAPT.
Aspirin
Aspirin can be given orally before primary PCI to ensure complete inhibition of thromboxane A2. The oral dose of plain aspirin (non-enteric-coated formulation) should be 150–300mg[15].
P2Y12 inhibitors
There is limited evidence regarding the timing of P2Y12 initiation in patients with STEMI[15]. Early initiation of a P2Y12 inhibitor (while the patient is being transported to a primary PCI centre) or at the time of primary PCI is recommended in Europe and the United States[5,15].
The Europe and Australia and New Zealand guidelines state that the preferred P2Y12 inhibitors are prasugrel (60mg loading dose) or ticagrelor (180mg loading dose)[15,16]. The US guideline is less explicit in its preference and provides an equal place in therapy for clopidogrel, prasugrel and ticagrelor[5]. In the recent update of the UK guideline, prasugrel was the P2Y12 inhibitor of choice in patients who are not on existing anticoagulation, while clopidogrel was the P2Y12 inhibitor of choice in those on existing anticoagulation[4].
Prasugrel and ticagrelor are superior to clopidogrel in onset of action, potency and clinical outcomes, such as reduction in the rate of death from vascular causes, MI or stroke[29]. The decision to use prasugrel as a first-line P2Y12 inhibitor in the National Institute for Health and Care Excellence (NICE) guideline was based largely on network meta-analysis of trials comparing prasugrel with clopidogrel, and ticagrelor with clopidogrel, and the ISAR-REACT 5 trial, which compared prasugrel directly with ticagrelor[30,31]. The guideline committee concluded that, overall, prasugrel was more effective than ticagrelor at 30 days and 1 year (with noteworthy differences for outcomes such as all-cause mortality and re-infarction)[4].
In the event that prasugrel and ticagrelor cannot be used owing to contraindications, all four international guidelines recommend the use of higher clopidogrel loading dose (600mg stat)[4,5,15,16]. Clopidogrel has not been evaluated against placebo in any large outcomes studies in primary PCI, but 600mg/150mg (loading dose/maintenance dose) in the first week was superior to the 300/75mg regimen in the subset of patients undergoing PCI in the CURRENT-OASIS-7 trial[32].
The other P2Y12 inhibitor cangrelor (intravenous) has limited use in current practice. European and UK guidelines recommend that cangrelor may be an alternative option for some people undergoing PCI, and for whom oral P2Y12 inhibitors are not feasible or desirable[4,15]. For example, in patients who are unable to absorb oral agents, patients with an unclear coronary anatomy such as aortic dissection, pericarditis or oesophageal tear, and patients in an unconscious state in whom bleeding risk is deemed to be low[33]. Cangrelor was not considered in the US guideline, and was not approved for use in Australia and New Zealand when their guideline was developed[5,16].
Anticoagulants
Anticoagulant options for primary PCI include unfractionated heparin (UFH), enoxaparin and bivalirudin. The choice of anticoagulant used in PCI differs between international guidelines. Heparin (UFH or enoxaparin) is recommended first-line in guidelines for Australia and New Zealand, the United States and Europe[5,15,16]. The UK guideline includes recommendations based on whether radial or femoral access was used[4]. For people undergoing PCI with radial access, UFH is preferred, while for those undergoing PCI with femoral access, bivalirudin is thought to be more suitable. NICE made the recommendation based on the availability of evidence that is most relevant for radial access and bailout glycoprotein inhibitor use[4]. The other guidelines examined also place bivalirudin as a second-line agent in patients with heparin-induced thrombocytopaenia or those with high risk of bleeding[5,15,16].
Enoxaparin is preferred over UFH as the heparin product for treatment in the European guideline[15]. This is largely owing to the superiority of enoxaparin over UFH in reducing mortality — especially in the primary PCI context — and is associated with reduction in major bleeding, as found in a meta-analysis of 23 PCI trials (30,966 patients, 33% primary PCI)[34]. The US guideline, however, recommends UFH as the heparin product of choice[5]. Additionally, UFH use in the US guideline is accompanied by activated partial thromboplastic time (aPTT), while the European guideline suggests that using aPTT to tailor dose or monitor UFH is thought to lack robust data in support[5,15]. Both guidelines considered the ATOLL trial, which compared intravenous enoxaparin with UFH[35]. While the primary composite endpoint of 30-day mortality, MI, procedural failure or major bleeding was not significantly reduced by enoxaparin (17% relative risk reduction, P=0.063), there was a reduction in the composite main secondary endpoint of death, recurrent MI or ACS, or urgent revascularisation[35] (the restoration of perfusion to the ischaemic region usually achieved by vascular bypass or angioplasty [if performed]). The European guideline considered this reduction in secondary endpoint, alongside no increase in bleeding, following the use of enoxaparin over UFH, which supported their decision to choose enoxaparin over UFH[15]. The Australia and New Zealand guideline did not specify a preference of heparin products[16].
Bivalirudin acts by binding directly to thrombin and has a more predictable anticoagulant effect compared to UFH. A meta-analysis of five trials that compared bivalirudin with UFH, with or without glycoprotein IIb/IIIa inhibitors, showed that bivalirudin was non-inferior to UFH in reducing mortality; superior in reducing risk of major bleeding; but inferior in risk of acute stent thrombosis[36]. The place in therapy of bivalirudin in the guidelines examined remains second-line for patients with high bleeding risk.
It is important to note that the use of fondaparinux in the context of primary PCI was associated with potential risk of stent thrombosis in the OASIS 6 trial [40] and is not recommended to be used in primary PCI[5,15].
Glycoprotein IIb/IIIa inhibitors
The glycoprotein IIb/IIIa inhibitors currently available on the UK market are eftifibatide and tirofiban. A meta-analysis of glycoprotein IIb/IIIa inhibition in ACS demonstrated a relative reduction in death or MI among patients undergoing PCI, but with an increased risk of major bleeding[37]. However, it is worth noting that evidence to support the use of intravenous glycoprotein IIb/IIIa receptor antagonists in patients with STEMI was established largely before the use of oral DAPT, therefore creating uncertainty around its place in contemporary practice[5]. The adjunctive use of glycoprotein IIb/IIIa agents at the time of PCI can be considered when there is evidence of large thrombus burden, ‘no-reflow’ or other thrombotic complications[5,15,16]. The routine adjunctive use of glycoprotein IIb/IIIa inhibitors for patients receiving bivalirudin as the primary anticoagulant is not recommended[5].
Pharmacotherapy for fibrinolysis
Antiplatelets
A loading dose of aspirin and clopidogrel should be administered to patients with STEMI who receive fibrinolytic therapy[5,15,16]. NICE recommends ticagrelor with aspirin for all patients with STEMI not treated with PCI (including those who received fibrinolysis), unless high bleeding risk exists[4]. For those with high bleeding risk, NICE recommends the use of either clopidogrel with aspirin or aspirin alone[4].
Clopidogrel added to aspirin reduces the risk of cardiovascular events and overall mortality in patients treated with fibrinolysis[38]. Aspirin should be continued indefinitely, and clopidogrel should be continued for at least 14 days and up to a year. Prasugrel and ticagrelor have not yet been studied as adjuncts to fibrinolysis[16]. While NICE acknowledged that direct evidence for the use of ticagrelor in patients not receiving PCI was lacking, the decision to recommend ticagrelor over clopidogrel for patients not treated with PCI in their guidelines was owed to the convincing superiority of ticagrelor in reducing mortality (cardiac and all-cause), and in preventing re-infarction and the need for future revascularisation procedures[4].
Anticoagulants
Parenteral anticoagulation should preferably be given until revascularisation. Otherwise, it should be given for at least 48 hours, or for the duration of the hospital stay, up to 8 days[5,15]. Both the European and US guidelines recommend the use of enoxaparin over UFH owing to the net clinical benefit of enoxaparin, which are based on the absence of death, non-fatal infarction and intracranial haemorrhage[5,15,39].
The large OASIS-6 trial demonstrated that fondaparinux was superior to placebo or UFH in preventing death and reinfarction, especially in patients who received streptokinase as a fibrinolytic agent[40]. The European guideline recommends fondaparinux in patients treated with streptokinase[15]. At the time of writing, fondaparinux had not been approved for use in Australia and New Zealand.
Bivalirudin is not recommended as an adjunct to fibrinolysis. The use of bivalirudin with streptokinase was found to be associated with a reduction in reinfarctions, but had an increased risk of bleeding[41]. The use of bivalirudin has not been studied with fibrin-specific agents; therefore, evidence to support the use of this combination is limited (see Figure).
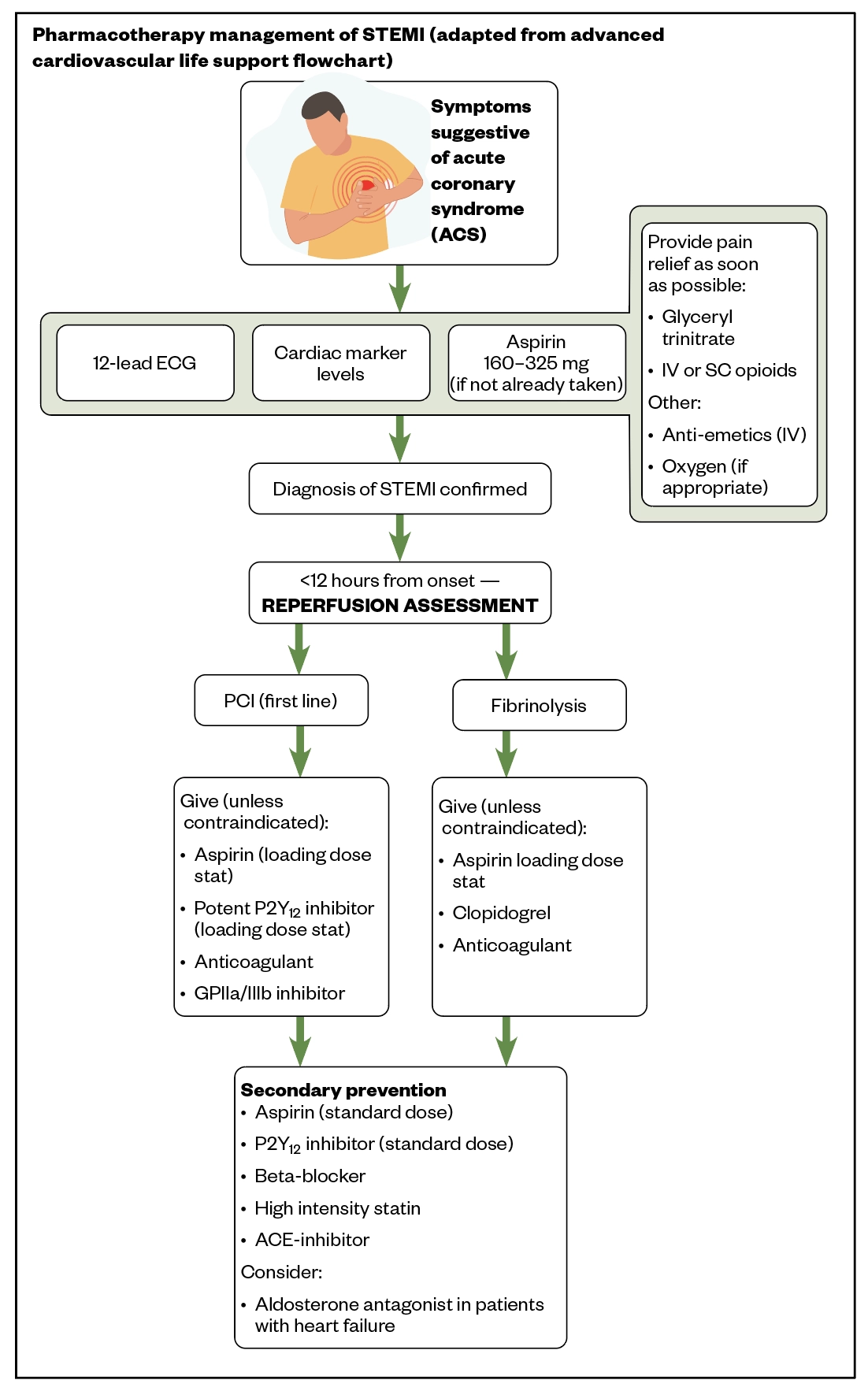
Source: ACLS Training Center[42]
STEMI: ST-elevation myocardial infarction; IV: intravenous; SC: subcutaneous
Secondary prevention in STEMI
The main goal of long-term maintenance pharmacotherapy is to prolong survival through risk factor modification and, ultimately, prevent further acute coronary episodes. Survivors of STEMI are at high risk of a recurrent MI, as well as other manifestations of cardiovascular disease, such as stroke, congestive cardiac failure and valve abnormalities[43]. Optimal medical therapy and lifestyle changes (e.g. diet, exercise and smoking cessation) are important in the prevention of recurrent ischaemic events[44].
Aspirin
Aspirin, used at a low maintenance dose, is recommended indefinitely in all patients with STEMI[4,5,15,16]. However, indefinite antiplatelet therapy with clopidogrel is recommended by both NICE and the Australia and New Zealand guidance in patients who have had prior stroke (not related to atrial fibrillation), patients with peripheral vascular disease or patients who cannot tolerate aspirin long-term[4,16].
P2Y12 inhibitors
In patients with confirmed STEMI, the use of a P2Y12 inhibitor at maintenance dose is recommended in addition to low-dose aspirin[4,5,15,16]. The Australia and New Zealand and European guidelines recommend the use of ticagrelor or prasugrel over clopidogrel, owing to the superiority of prasugrel and ticagrelor over clopidogrel in reducing mortality, recurrent MI and stroke in patients with intermediate or very high risk ACS[15,16,29,45]. However, in patients aged over 75 years, or of low body weight (<60 kg) or previous cerebrovascular disease, prasugrel was associated with more harm than benefit when compared with clopidogrel[16]. Clopidogrel is recommended for patients who cannot receive ticagrelor or prasugrel as an adjunctive agent with fibrinolysis, or for those requiring oral anticoagulation[15,16].
Duration of dual anti-platelet therapy
DAPT, combining aspirin and a P2Y12 inhibitor, is recommended in all guidelines in patients with STEMI who have undergone primary PCI for up to 12 months[4,5,15,16]. Clopidogrel should be used for one month in patients treated with fibrinolysis without subsequent PCI; however, European and UK guidelines recommend that an extension of DAPT duration to 12 months should be considered in these patients[4,5,15,38]. For patients undergoing fibrinolysis and subsequent PCI, DAPT is recommended for 12 months[4,15]. Clopidogrel is the P2Y12 inhibitor of choice as a co-adjuvant and after fibrinolysis[15].
In patients with high bleeding risk, studies have shown that shortening DAPT duration to 6 months, compared with 12 months or longer, reduces the risk of major bleeding complications with no apparent trade-off in ischaemic events[46].
In contrast, two major studies have shown the benefit towards reduction of non-fatal ischaemic events in patients receiving longer than 12 months of DAPT[47,48]. Currently, the use of ticagrelor beyond one year (up to three years) is recommended as an option in patients with high ischaemic risk[15,16,49]. The use of clopidogrel beyond one year as part of a DAPT regimen is recommended by the Australia and New Zealand guideline, based on a DAPT study, while it is not recommended by the European guideline[15,16,48]. The European guideline states that because the DAPT study only included around 10% of STEMI patients, and no information has so far been provided on the benefit of prolonging clopidogrel from 12–30 months in this patient subset, no formal recommendations are possible for the use of clopidogrel or prasugrel beyond one year[15,48]. The use of DAPT beyond one year (up to three years) in the form of aspirin plus ticagrelor 60mg twice per day was supported by data from the PEGASUS-TIMI 54 trial[47]. The use of DAPT may sometimes be indicated in addition to long-term oral anticoagulation in patients with conditions, such as atrial fibrillation[50]. European guidelines estimate that around 6–8% of patients undergoing PCI have an indication for long-term oral anticoagulation[51]. This is referred to as ‘triple therapy’ (i.e. DAPT and oral anticoagulation); however, as this increases bleeding risk, its duration should be kept to the minimum required for maximum benefit/risk ratio. The recommended duration of triple therapy is highly dependent on the individual bleeding risk versus clotting risk factors of the patient[4]. In the absence of safety and efficacy data, the use of prasugrel or ticagrelor as part of triple therapy should be avoided[51]. Clopidogrel is recommended where the use of triple therapy is required and a co-prescription with a proton pump inhibitor is recommended for gastroprotection to help reduce the risk of gastrointestinal bleeding[4,15,16].
Beta-blockers
Beta-blockers should be started within the first 24 hours of the presentation of chest pain in patients who are haemodynamically stable (i.e. where blood pressure and cardiac output are thought to be sufficient in maintaining normal organ functions)[15]. Beta-blockers reduce myocardial oxygen consumption by lowering heart rate, blood pressure and myocardial contractility.
After early intravenous beta blocker treatment (e.g. metoprolol), patients should be continued on oral vasodilatory beta-blocker therapy long term, unless contraindicated[4,5,15]. Vasodilatory beta-blockers include carvedilol, bisoprolol, nebivolol and metoprolol, and reduce peripheral vascular resistance while maintaining or improving cardiac output, stroke volume and left ventricular function. They also may limit infarct size[52].
The benefit of long-term treatment with oral beta-blockers after STEMI is well established, although most of the supporting data are from trials performed in the pre-reperfusion era[53]. Evidence supporting the use of beta-blockers is strong among patients with reduced left ventricular function following ACS. However, most of the benefit occurs within the first year of acute MI, with benefit beyond one year being less evident. Therefore, in patients with MI without underlying heart failure or hypertension, the US guideline recommends that beta-blocker use be limited to a three-year course[5]. However, NICE and Australia and New Zealand guidelines recommend that beta-blocker use in this cohort of patients (without underlying heart failure) should be reviewed after 12 months with a view to stopping treatment, taking into account the extent of coronary disease or evidence of ischaemia, concurrent conditions and any adverse effects[4,16]. The European guideline states that, given the absence of studies that appropriately addressed beta-blocker duration to date, no recommendation could be made in this respect[15].
Lipid modification therapy
All guidelines recommend that high-intensity statin (i.e. statin regimens that reduce low-density lipoprotein cholesterol [LDL-C] by around 50%) should be initiated as soon as the patient is stabilised and has no contraindications, and should be continued long term[4,5,15,16]. Treatment with statins in patients stabilised after an ACS, including STEMI, lowers the risk of coronary heart disease death, recurrent MI, stroke and the need for coronary revascularisation[54]. A meta-analysis of trials comparing more versus less intensive LDL-C lowering with statins indicated that more intensive statin therapy produced greater reductions in the risks of cardiovascular death, non-fatal MI, ischaemic stroke, and coronary revascularisation[55]. Among currently available statins, only high-dose atorvastatin (80mg daily) has been shown to reduce death and ischemic events among patients with ACS[56,57]. Treatment with statins is irrespective of cholesterol concentration at presentation.
In patients known to be intolerant of any dose of statin, or who may have suboptimal LDL cholesterol levels despite statin treatment, treatment with ezetimibe should be considered, as recommended by the European and the Australia and New Zealand guidelines[15,16]. This recommendation was largely based on the In the Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT), where 18,144 patients with a recent ACS (29% with STEMI) were randomised to either ezetimibe plus simvastatin or simvastatin alone, and found that ezetimibe plus simvastatin produced a statistically significant reduction in the composite outcome of cardiovascular death, MI, hospital admission for unstable angina, coronary revascularisation or stroke[58].
Protein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) are recommended as second or third line lipid lowering agents by international guidelines[59,60]. By inhibiting the PCSK9 protein, PCSK9i can lower LDL-C levels by enabling the liver to metabolise it. Furthermore, the FOURIER trial demonstrated that in 27,564 high-risk patients with pre-existing CVD whose LDL-C levels were at least 70mg/dL (1.8mmol/L) despite optimised statin therapy, the addition of evolocumab (a PCSK9i) to a statin resulted in a relative risk reduction of 15% in primary composite endpoints of cardiovascular death, MI, stroke, hospitalisation for unstable angina or coronary revascularisation, compared with those treated with placebo[61]. In view of this, there may be further scope for the use of PCSK9i in high-risk patients whose cholesterol levels cannot be lowered by statins alone. More specific trials are currently underway using PCSK9 inhibitors in high risk patients with ACS (EVOPACS using evolocimab and ORION-4 trial using inclisiran)[62,63].
Renin-angiotensin system inhibitors
Oral angiotensin-converting enzyme (ACE) inhibitors reduce fatal and non-fatal major cardiovascular events in patients with STEMI[64]. Their protective effects have been demonstrated independent of the use of other pharmacotherapies (i.e. fibrinolytics, aspirin and beta blockers); with the greatest clinical benefit seen in high-risk patient subgroups (i.e. anterior MI, ejection fraction ≤ 40%, heart failure, prior MI, and tachycardia)[65]. The benefit of early initiation of these agents in patients with MI is evident and is therefore recommended by all four guidelines for patients without existing contraindications[4,5,15,16]. In patients who cannot tolerate ACE inhibitors, angiotensin receptor blockers (ARBs) are indicated[4,5,15,16]. Valsartan is the preferred ARB owing to the VALIANT (Valsartan in Acute Myocardial Infarction) trial, where it was found to be non-inferior to captopril[5,15,64].
Aldosterone antagonist
An aldosterone antagonist is recommended in patients with left ventricular dysfunction (left ventricular ejection fraction ≤40%) and heart failure after STEMI[4,5,15]. Eplerenone is the only aldosterone antagonist with evidence for use in this cohort of patients at the time of writing[66]. The initiation of eplerenone within seven days of MI significantly reduced the rates of all-cause mortality, sudden cardiac death and cardiovascular mortality/hospitalisation, whereas initiation after seven days had no significant effect on the above mentioned outcomes[67].
Conclusion
While the four clinical guidelines were from countries/regions with a similar demographic and disease burden to the UK, this review has highlighted that there are several differences across them in relation to the pharmacotherapy and interventions of STEMI.
First, as primary PCI is the treatment of choice for STEMI, provided it could be delivered ‘in a timely fashion’, it was interesting to see that variation exists between the guidelines on the recommended time to reperfusion, with the shortest recommended time of within 90 minutes to up to 120 minutes in the UK and European guidelines[4,15,16]. However, most centres in the UK achieved ‘door-to-balloon’ (DTB) time of under 90 minutes[19]. It is unclear how the UK compares with the other countries on this performance measure as international comparisons are difficult in this area of practice, owing to the different healthcare systems and the different targets reported. Importantly, as identified in the European guideline and its review of the literature, there is still a lack of data on the definitive upper threshold of time to reperfusion and the lack of international comparative data on prognosis[68]. More research is recommended in this area: to agree and identify standardised DTB time to allow for international comparisons to be made, and exploration into its link with prognosis and the ‘gold standard’ health model, to allow for quicker patient access to primary PCI.
Second, all guidelines examined specified the preference of using prasugrel or ticagrelor as the P2Y12 inhibitor of choice in reperfusion, other than the US guideline, which did not specify a preferred agent[5]. However, evidence has shown the superiority of prasugrel and ticagrelor over clopidogrel in rapid onset of action, potency, and clinical outcomes[29,45]. While having no preferred agent offers a choice of P2Y12 inhibitors, where superior outcome data exists, clinical recommendations should reflect that.
Another variation in recommendation was in the choice of heparin used during primary PCI: the United States recommended UFH, while Europe recommended enoxaparin[5,15]. Both guidelines derived their recommendations from the same study: the ATOLL study, where the United States considered only the primary endpoint, while Europe took into consideration the secondary endpoint as well as its harm data[5,15,35]. However, as a large meta-analysis study showing the superiority of enoxaparin over UFH was not considered by the US guideline (owing to publication time being close together), there is a possibility that recommendations may reflect that of Europe’s once an update is carried out[15,34].
Next, the use of clopidogrel, as part of extended DAPT beyond one year, was recommended by the Australia and New Zealand guideline, but was found to have insufficient evidence to be recommended by the European guideline[15,16]. NICE and the American College of Cardiology did not make any specific recommendations around this[4,5]. It was widely acknowledged by the guidelines that there are still significant uncertainties in the evidence around antiplatelet use: specifically around the safety and efficacy of ‘triple therapy’ (direct oral anticoagulants and dual antiplatelets); the optimal duration of maintenance therapy with P2Y12 inhibitors as a single or multiple antiplatelet regimens; and the role of aspirin in the new era of potent antiplatelet agents and low dose anticoagulation[4,5,15,16].
Lastly, this review also found variations in guidelines’ recommendations on the duration of use of beta-blocker therapy in patients without heart failure and/or low left ventricular ejection fraction, with the recommended duration ranging from 12 months to 3 years[4,5,16]. The lack of evidence for use of beta-blockers in the post-reperfusion era creates a research gap to establish its clinical value for this cohort of patients. While it is acceptable to keep patients on this treatment, providing there is no harm, it may have a detrimental effect on the wellbeing of patients owing to the pill burden associated with it.
The use of PCSK9 inhibitors is an area of emerging research, with likely updates to guidance expected in the near future.
- 1Myocardial infarction redefined—A consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the Redefinition of Myocardial Infarction. European Heart Journal 2000;21:1502–13. doi:10.1053/euhj.2000.2305
- 2Lippi G, Sanchis-Gomar F, Cervellin G. Chest pain, dyspnea and other symptoms in patients with type 1 and 2 myocardial infarction. A literature review. International Journal of Cardiology 2016;215:20–2. doi:10.1016/j.ijcard.2016.04.045
- 3Ambrose JA, Singh M. Pathophysiology of coronary artery disease leading to acute coronary syndromes. F1000Prime Rep 2015;7. doi:10.12703/p7-08
- 4NG185: Acute coronary syndromes. National Institute for Health and Care Excellence. 2020.https://www.nice.org.uk/guidance/ng185 (accessed Jun 2021).
- 5O’Gara PT, Kushner FG, et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. Circulation 2013;127. doi:10.1161/cir.0b013e3182742cf6
- 6Cardiovascular disease statistics. British Heart Foundation. 2014.https://www.bhf.org.uk/informationsupport/publications/statistics/cardiovascular-disease-statistics-2014 (accessed Jun 2021).
- 7Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation 2017;135. doi:10.1161/cir.0000000000000485
- 8Grech ED. Acute coronary syndrome: ST segment elevation myocardial infarction. BMJ 2003;326:1379–81. doi:10.1136/bmj.326.7403.1379
- 9Myocardial infarction. Medscape. 2019.https://emedicine.medscape.com/article/155919-overview (accessed Jun 2021).
- 10Fox KAA. British Cardiac Society Working Group on the definition of myocardial infarction. Heart 2004;90:603–9. doi:10.1136/hrt.2004.038679
- 11Setiadi B, Lei H, Chang J. Troponin not just a simple cardiac marker: prognostic significance of cardiac troponin. Chin Med J (Engl) 2009;122:351–8.https://www.ncbi.nlm.nih.gov/pubmed/19236818
- 12Kikkert WJ, van Geloven N, van der Laan MH, et al. The Prognostic Value of Bleeding Academic Research Consortium (BARC)-Defined Bleeding Complications in ST-Segment Elevation Myocardial Infarction. Journal of the American College of Cardiology 2014;63:1866–75. doi:10.1016/j.jacc.2014.01.069
- 13Cenko E, Yoon J, Kedev S, et al. Sex Differences in Outcomes After STEMI. JAMA Intern Med 2018;178:632. doi:10.1001/jamainternmed.2018.0514
- 14Verma VK, Hollenberg SM. Update on acute coronary syndromes and ST-elevation myocardial infarction. Current Opinion in Internal Medicine 2005;4:614–8. doi:10.1097/01.ccx.0000176697.77636.3a
- 15Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. European Heart Journal 2017;39:119–77. doi:10.1093/eurheartj/ehx393
- 16Chew DP, Scott IA, Cullen L, et al. National Heart Foundation of Australia & Cardiac Society of Australia and New Zealand: Australian Clinical Guidelines for the Management of Acute Coronary Syndromes 2016. Heart, Lung and Circulation 2016;25:895–951. doi:10.1016/j.hlc.2016.06.789
- 17Borzak S MD, Cannon CP MD, Kraft PL MD, et al. Effects of Prior Aspirin and Anti-Ischemic Therapy on Outcome of Patients With Unstable Angina 11This study was supported by Biogen, Inc., Cambridge, Massachusetts. Manuscript received July 28, 1997; revised manuscript received and accepted December 1, 1997. The American Journal of Cardiology 1998;81:678–81. doi:10.1016/s0002-9149(97)01006-0
- 18Parodi G, Bellandi B, Xanthopoulou I, et al. Morphine Is Associated With a Delayed Activity of Oral Antiplatelet Agents in Patients With ST-Elevation Acute Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Circ Cardiovasc Interv 2015;8. doi:10.1161/circinterventions.114.001593
- 19National audit of percutaneous coronary interventions annual public report. Healthcare Quality Improvement Partnership. 2017.https://www.hqip.org.uk/resource/national-audit-of-percutaneous-coronary-intervention-annual-public-report/ (accessed Jun 2021).
- 20Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. The Lancet 2003;361:13–20. doi:10.1016/s0140-6736(03)12113-7
- 21Serruys PW, Ormiston JA, Onuma Y, et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. The Lancet 2009;373:897–910. doi:10.1016/s0140-6736(09)60325-1
- 22Riley RF, Don CW, Powell W, et al. Trends in Coronary Revascularization in the United States From 2001 to 2009. Circ Cardiovasc Qual Outcomes 2011;4:193–7. doi:10.1161/circoutcomes.110.958744
- 23Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Lancet 1994;343:311–22.https://www.ncbi.nlm.nih.gov/pubmed/7905143
- 24Boersma E, Maas AC, Deckers JW, et al. Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour. The Lancet 1996;348:771–5. doi:10.1016/s0140-6736(96)02514-7
- 25Pinto DS, Frederick PD, Chakrabarti AK, et al. Benefit of Transferring ST-Segment–Elevation Myocardial Infarction Patients for Percutaneous Coronary Intervention Compared With Administration of Onsite Fibrinolytic Declines as Delays Increase. Circulation 2011;124:2512–21. doi:10.1161/circulationaha.111.018549
- 26An International Randomized Trial Comparing Four Thrombolytic Strategies for Acute Myocardial Infarction. N Engl J Med 1993;329:673–82. doi:10.1056/nejm199309023291001
- 27Martin C, Sobolewski K, Bridgeman P, et al. Systemic Thrombolysis for Pulmonary Embolism: A Review. P T 2016;41:770–5.https://www.ncbi.nlm.nih.gov/pubmed/27990080
- 28Brouwer MA. Adjunctive treatment in patients treated with thrombolytic therapy. Heart 2004;90:581–8. doi:10.1136/hrt.2003.019877
- 29Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N Engl J Med 2009;361:1045–57. doi:10.1056/nejmoa0904327
- 30Acute Coronary Syndromes: [A] Evidence review for antiplatelet therapy. National Institute for Health and Care Excellence. 2020.https://www.nice.org.uk/guidance/ng185/documents/evidence-review (accessed May 2021).
- 31Schüpke S, Neumann F-J, Menichelli M, et al. Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes. N Engl J Med 2019;381:1524–34. doi:10.1056/nejmoa1908973
- 32Mehta SR, Tanguay J-F, Eikelboom JW, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. The Lancet 2010;376:1233–43. doi:10.1016/s0140-6736(10)61088-4
- 33Evidence summary [ESNM63]: Coronary revascularisation — Cangrelor. National Institute for Health and Care Excellence. 2015.https://www.nice.org.uk/guidance/cg95 (accessed May 2021).
- 34Silvain J, Beygui F, Barthelemy O, et al. Efficacy and safety of enoxaparin versus unfractionated heparin during percutaneous coronary intervention: systematic review and meta-analysis. BMJ 2012;344:e553–e553. doi:10.1136/bmj.e553
- 35Montalescot G, Zeymer U, Silvain J, et al. Intravenous enoxaparin or unfractionated heparin in primary percutaneous coronary intervention for ST-elevation myocardial infarction: the international randomised open-label ATOLL trial. The Lancet 2011;378:693–703. doi:10.1016/s0140-6736(11)60876-3
- 36Capodanno D, Gargiulo G, Capranzano P, et al. Bivalirudin versus heparin with or without glycoprotein IIb/IIIa inhibitors in patients with STEMI undergoing primary PCI: An updated meta-analysis of 10,350 patients from five randomized clinical trials. European Heart Journal: Acute Cardiovascular Care 2015;5:253–62. doi:10.1177/2048872615572599
- 37Bosch X, Marrugat J, Sanchis J. Platelet glycoprotein IIb/IIIa blockers during percutaneous coronary intervention and as the initial medical treatment of non-ST segment elevation acute coronary syndromes. Cochrane Database of Systematic Reviews. 2010. doi:10.1002/14651858.cd002130.pub2
- 38Addition of clopidogrel to aspirin in 45 852 patients with acute myocardial infarction: randomised placebo-controlled trial. The Lancet 2005;366:1607–21. doi:10.1016/s0140-6736(05)67660-x
- 39Giraldez RR, Nicolau JC, Corbalan R, et al. Enoxaparin is superior to unfractionated heparin in patients with ST elevation myocardial infarction undergoing fibrinolysis regardless of the choice of lytic: an ExTRACT-TIMI 25 analysis. European Heart Journal 2007;28:1566–73. doi:10.1093/eurheartj/ehm179
- 40Effects of Fondaparinux on Mortality and Reinfarction in Patients With Acute ST-Segment Elevation Myocardial Infarction: The OASIS-6 Randomized Trial. JAMA: The Journal of the American Medical Association 2006;295:1519–30. doi:10.1001/jama.295.13.joc60038
- 41Thrombin-specific anticoagulation with bivalirudin versus heparin in patients receiving fibrinolytic therapy for acute myocardial infarction: the HERO-2 randomised trial. The Lancet 2001;358:1855–63. doi:10.1016/s0140-6736(01)06887-8
- 42Acute coronary syndrome algorithm. ACLS Training Center. 2017.https://www.acls.net/acute-coronary-syndromes-algorithm.htm (accessed Jun 2021).
- 43Smolina K, Wright FL, Rayner M, et al. Long-Term Survival and Recurrence After Acute Myocardial Infarction in England, 2004 to 2010. Circ Cardiovasc Qual Outcomes 2012;5:532–40. doi:10.1161/circoutcomes.111.964700
- 44Chow CK, Jolly S, Rao-Melacini P, et al. Association of Diet, Exercise, and Smoking Modification With Risk of Early Cardiovascular Events After Acute Coronary Syndromes. Circulation 2010;121:750–8. doi:10.1161/circulationaha.109.891523
- 45Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus Clopidogrel in Patients with Acute Coronary Syndromes. N Engl J Med 2007;357:2001–15. doi:10.1056/nejmoa0706482
- 46Costa F, Tijssen JG, Ariotti S, et al. Incremental Value of the CRUSADE, ACUITY, and HAS‐BLED Risk Scores for the Prediction of Hemorrhagic Events After Coronary Stent Implantation in Patients Undergoing Long or Short Duration of Dual Antiplatelet Therapy. JAHA 2015;4. doi:10.1161/jaha.115.002524
- 47Bonaca MP, Bhatt DL, Cohen M, et al. Long-Term Use of Ticagrelor in Patients with Prior Myocardial Infarction. N Engl J Med 2015;372:1791–800. doi:10.1056/nejmoa1500857
- 48Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 Months of Dual Antiplatelet Therapy after Drug-Eluting Stents. N Engl J Med 2014;371:2155–66. doi:10.1056/nejmoa1409312
- 49NICE Technology appraisal TA420: Ticagrelor for preventing atherothrombotic events after myocardial infarction. National Institute for Health and Care Excellence. 2016.https://www.nice.org.uk/guidance/ta420 (accessed May 2021).
- 50Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the Management of Atrial Fibrillation Developed in Collaboration With EACTS. Revista Española de Cardiología (English Edition) 2017;70:50. doi:10.1016/j.rec.2016.11.033
- 51Roffi M, Patrono C, Collet J-P, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2015;37:267–315. doi:10.1093/eurheartj/ehv320
- 52Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. The Lancet 2001;357:1385–90. doi:10.1016/s0140-6736(00)04560-8
- 53Freemantle N, Cleland J, Young P, et al. beta Blockade after myocardial infarction: systematic review and meta regression analysis. BMJ 1999;318:1730–7. doi:10.1136/bmj.318.7200.1730
- 54Sacks FM, Pfeffer MA, Moye LA, et al. The Effect of Pravastatin on Coronary Events after Myocardial Infarction in Patients with Average Cholesterol Levels. N Engl J Med 1996;335:1001–9. doi:10.1056/nejm199610033351401
- 55Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. The Lancet 2010;376:1670–81. doi:10.1016/s0140-6736(10)61350-5
- 56Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med 2015;372:2387–97. doi:10.1056/nejmoa1410489
- 57Schwartz GG. Effects of Atorvastatin on Early Recurrent Ischemic Events in Acute Coronary Syndromes<SUBTITLE>The MIRACL Study: A Randomized Controlled Trial</SUBTITLE>. JAMA 2001;285:1711. doi:10.1001/jama.285.13.1711
- 58Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus Moderate Lipid Lowering with Statins after Acute Coronary Syndromes. N Engl J Med 2004;350:1495–504. doi:10.1056/nejmoa040583
- 59Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2017 Focused Update of the 2016 ACC Expert Consensus Decision Pathway on the Role of Non-Statin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk. Journal of the American College of Cardiology 2017;70:1785–822. doi:10.1016/j.jacc.2017.07.745
- 60Landmesser U, Chapman MJ, Stock JK, et al. 2017 Update of ESC/EAS Task Force on practical clinical guidance for proprotein convertase subtilisin/kexin type 9 inhibition in patients with atherosclerotic cardiovascular disease or in familial hypercholesterolaemia. European Heart Journal 2017;39:1131–43. doi:10.1093/eurheartj/ehx549
- 61Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med 2017;376:1713–22. doi:10.1056/nejmoa1615664
- 62Koskinas KC, Windecker S, Pedrazzini G, et al. Evolocumab for Early Reduction of LDL Cholesterol Levels in Patients With Acute Coronary Syndromes (EVOPACS). Journal of the American College of Cardiology 2019;74:2452–62. doi:10.1016/j.jacc.2019.08.010
- 63Stoekenbroek RM, Kallend D, Wijngaard PL, et al. Inclisiran for the treatment of cardiovascular disease: the ORION clinical development program. Future Cardiology 2018;14:433–42. doi:10.2217/fca-2018-0067
- 64Pfeffer MA, Braunwald E, Moyé LA, et al. Effect of Captopril on Mortality and Morbidity in Patients with Left Ventricular Dysfunction after Myocardial Infarction. N Engl J Med 1992;327:669–77. doi:10.1056/nejm199209033271001
- 65Dickstein K, Kjekshus J. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. The Lancet 2002;360:752–60. doi:10.1016/s0140-6736(02)09895-1
- 66Pitt B, Remme W, Zannad F, et al. Eplerenone, a Selective Aldosterone Blocker, in Patients with Left Ventricular Dysfunction after Myocardial Infarction. N Engl J Med 2003;348:1309–21. doi:10.1056/nejmoa030207
- 67Adamopoulos C, Ahmed A, Fay R, et al. Timing of eplerenone initiation and outcomes in patients with heart failure after acute myocardial infarction complicated by left ventricular systolic dysfunction: insights from the EPHESUS trial†. European Journal of Heart Failure 2009;11:1099–105. doi:10.1093/eurjhf/hfp136
- 68Chung S-C, Sundström J, Gale CP, et al. Comparison of hospital variation in acute myocardial infarction care and outcome between Sweden and United Kingdom: population based cohort study using nationwide clinical registries. BMJ 2015;:h3913. doi:10.1136/bmj.h3913