
Shutterstock.com
After reading this article, you should be able to:
- Understand the role of the pharmacist in supporting people with depression;
- Explain the diagnostic criteria for depression;
- Know which depression screening tools can be incorporated into your practice;
- Know which evidenced-based pharmacological and non-pharmacological treatment options are available for depression;
- Know the essential counselling points for starting antidepressant medication;
- Understand the risk and distinguishing features of serotonin syndrome as a potential side effect of antidepressant use.
The prevalence of depression worldwide averages around 5% and appears to be higher still in developed countries[1,2]. A 2022 survey of the British adult population estimated that, in any given week, one in six people were experiencing symptoms of depression and only a small minority were receiving treatment[3]. This is concerning as untreated depression has serious consequences; it can increase the chance of risky behaviours, and create problems in relationships and at work. Depression therefore carries a high socioeconomic burden[1,3]. Pharmacists are likely to encounter many people with untreated depression in their daily practice — by supporting patients to access treatment earlier and signposting them for diagnosis, they are in a position to reduce the impact on individuals and families, and lessen the economic cost to society[4,5].
This article considers how to identify and screen for undiagnosed depression and explores the full range of treatment and management recommendations for depression, as outlined in recently updated guidance from the National Institute for Health and Care Excellence (NICE)[6]. It also considers the role of medication in the treatment of depression and how pharmacists can support patients with adherence and ongoing monitoring of side effects and changes in symptoms.
Diagnostic criteria, signs and symptoms
Causes of depression are often multifactorial and it may be difficult to identify a trigger in some cases[7]. All cases of depression, regardless of severity, share the same cardinal feature of a loss of positive affect. The individual may experience this as persistent feelings of sadness (depressed mood), a lack of “joy” from more pleasurable aspects of life (i.e. anhedonia), or both[8]. These are classed as core symptoms of depression in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V)[9].
Additional symptoms should also be present — for example, suicidality, feelings of guilt or worthlessness, difficulty concentrating, appetite changes, unexplained tiredness and sleep disturbance — with the number of symptoms and their impact on the individual’s wellbeing dictating the severity of the episode[10].
Note that, for a diagnosis of depression, a total of five or more of the above symptoms must be present, including at least one core symptom[9]. Additionally, these symptoms must:
- Be present over a period of at least two weeks;
- Have caused significant distress to the individual during this time and/or significant functional impairment[9,11].
Screening for depression
In its early stages, depression is highly treatable but, without timely intervention, symptoms may worsen to the extent that an individual experiences significant functional deficits, including difficulties maintaining self-care, meaningful relationships and employment. Those worst affected may try to take their own life[1,4]. It is therefore imperative that depression is identified promptly.
As many symptoms of depression are not visible (e.g. thoughts of suicidality), incorporating screening tools into practice can be helpful[5]. For example, primary care network (PCN) pharmacy teams are often tasked with conducting structured medication reviews in patients with a chronic physical health condition(s) or polypharmacy[12]. As these patients have a higher risk of developing depression, this presents an opportunity to support those found to be experiencing depressive symptoms with accessing timely treatment[13,14].
NICE recommends using two screening questions to uncover potential depressive symptoms in patients at higher risk (i.e. those with physical health conditions). These two screening questions, shown in Box 1, are based on the core symptoms of depression from the DSM-V[9]. Those patients answering “yes” to one or both screening questions should be referred to a GP or specialist practitioner for assessment[15].
Box 1: Screening questions for depression
- During the past month, have you often been bothered by feeling down, depressed or hopeless?
- During the past month, have you often been bothered by having little interest or pleasure in doing things?[6]
Another simple, brief intervention screening tool for depression is the Patient Health Questionnaire-2 (PHQ-2). It is an extraction of two questions from the Patient Health Questionnaire (PHQ)[9,16,17].
The table shows how the PHQ-2 questionnaire is scored. Similar to the NICE screening questions in Box 1, PHQ-2 scans for the presence of the two core symptoms of depression (anhedonia, depressed mood) but over a shorter time period[17].
The binary scoring system (below 3 = depression unlikely; 3 or above = depression possible) makes this brief depression screening instrument ideal for incorporating into standard structured medication review templates[14]. Patients scoring 3 or above on the PHQ-2 will have experienced at least one core symptom of depression at least half of the time and found it bothersome; therefore, there is a good chance they may be experiencing other symptoms of depression.
A diagnosis may be supported or refuted by administering the full PHQ-9, which screens for the presence, frequency, and severity of all core and ancillary depressive symptoms listed in the DSM-V[11,17]. Diagnosis should ideally be confirmed by a clinician, though NICE does endorse the use of the PHQ-9 as a tool to evaluate the severity of the depressive episode, its treatment (see NICE treatment recommendations, below), and the patient’s response to treatment. Clinicians may assess the impact of any intervention by comparing the PHQ-9 score at subsequent review points with the pre-treatment score[14].
Screening calculators derived from each questionnaire are easily accessible online (see Further reading for links to examples) and are often embedded into electronic patient record systems[18].
NICE suggests that individuals scoring 16 or above on the PHQ-9 with a confirmed diagnosis of depression are treated for ‘more severe’ depression (formerly called ‘moderate-to-severe depressive symptoms’), while those scoring less than 16 on PHQ-9 should be treated for ‘less severe’ depression (previously ‘subthreshold or mild-to-moderate symptoms’)[6].
Treating depression
Depression may respond to a variety of management options. The figure below summarises these options in order of their cost-effectiveness and ease of implementation, depending on the severity of the episode[19].
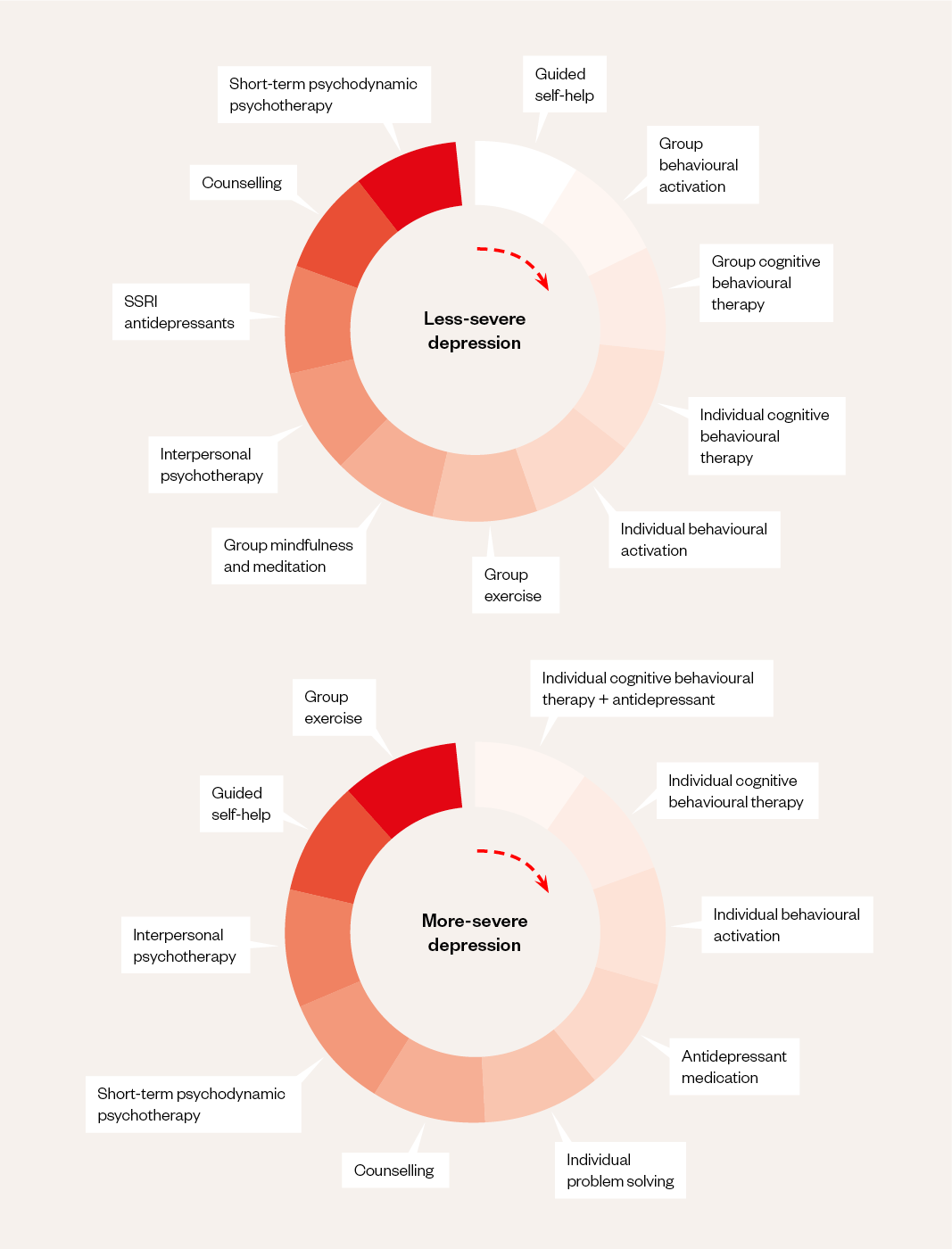
SSRI: selective serotonin reuptake inhibitor
Treatment should be tailored to the individual’s unique circumstances and preferences, rather than blanket prescribing of the most cost-effective option for the subtype of depression. For instance, some patients may express a preference for talking therapies, while others may prefer medication.
When choosing an antidepressant, patients who struggle with adherence when depressed may be more at risk of experiencing antidepressant withdrawal syndrome when prescribed medication with a short half-life. They may therefore benefit from a once-daily dosing regimen and rationalisation of other prescribed medications to support treatment adherence[15].
Taking a history from the patient and gaining insight into their perspective and expectations of treatment is therefore an important first step[20]. NICE recommends covering the following points with the patient:
- Factors the patient believes could be contributing to or exacerbating their depression;
- Their thoughts or preferences about starting treatment;
- Any previous episodes of depression and treatments tried;
- Their expectations of treatment[6].
Readers are referred to Table 1 (covering less severe depression) and Table 2 (covering more severe depression) in the NICE guideline for an overview of each management option and their respective risks and benefits[5]. For instance, when selecting medication as a treatment option for more severe depression, Table 2 advises prescribing tricyclic antidepressants (TCAs) with caution, owing to their toxicity in overdose[6]. Pharmacists may find these tables useful when comparing non-pharmacological interventions to treatment with an antidepressant[15].
Barriers to accessing treatment
Although NICE places a responsibility on commissioners to ensure all first-line treatment options for depression are available, in practice, individual or system barriers may dictate which options are truly accessible[21]. For example, communication or engagement difficulties, workforce capacity issues and local commissioning arrangements could all lead to delays in patients accessing treatment[22].
Explaining all the available treatment options to the patient and discussing the individual’s expectations of treatment will inevitably entail longer consultation times. However, both clinician and patient stakeholders believe investing this time prior to commencing treatment leads to improved outcomes[15]. Shared decision-making and patient-centred care can both help to support adherence to treatment, whether this be taking medication as prescribed, committing to behavioural activation exercises or consistently attending group sessions[23].
Healthcare professionals should consider their own internal biases when selecting treatment for patients in stigmatised groups, such as those with a comorbid personality disorder, psychosis or chronic depression, who may be seen as more difficult to treat. In its guidance, NICE emphasises that these often-stigmatised groups have the same rights to care and treatment as other individuals[15].
Note that presenting a menu of options may be overwhelming for some people with depression, who may experience problems with concentration or psychomotor disturbance (e.g. agitation)[24,25]. These symptoms may grossly interfere with their ability to process and comprehend the full range of treatment options[26].
A patient who is feeling overwhelmed may ask to end the consultation early, decline treatment (see Box 2 for guidance on how to follow up with patients who decline treatment) or ask the clinician to choose a treatment on their behalf. In that case, it is important to respect their wishes[27]. Some patients may require several follow-up consultations to cover the full range of options, but where faster intervention is required — for instance, where suicidality is present — it would seem reasonable to offer the most cost-effective and clinically appropriate option for that individual.
NICE recommends either selecting the least intrusive and resource-efficient option for a patient’s clinical needs, or an option that has helped them in the past[15]. If a patient with less severe depression declines any offers of treatment, remember that this is also a choice and offer appropriate follow-up.
Box 2: Patients who decline offers of treatment
For people with less severe depression who do not want treatment, or people who feel that their depressive symptoms are improving:
- Discuss the presenting problem(s) and any underlying vulnerabilities and risk factors, as well as any concerns that the person may have;
- Ensure the person knows they can change their mind and how to seek help;
- Provide information about the nature and course of depression;
- Arrange a further assessment, normally within two to four weeks;
- Make contact (with repeated attempts if necessary) if the person does not attend follow-up appointments[6].
Additional considerations
Inviting a trusted friend or relative of the patient to the consultation, with their consent, and offering written information are both helpful adaptations for people with depression, who may struggle to fully recall the discussion later owing to the nature of their symptoms.
Where medication forms part of the treatment plan, and the patient has previously received treatment under the Mental Health Act, they should be invited to make an advance statement. This entails documenting their wishes and preferences for treatment — including treatments they wish to avoid — should they become severely unwell. Patients should also be informed of their right to appoint a health and welfare lasting power of attorney, who may advocate for them should they lose capacity to make an informed decision at any point[6,28].
Role of medication in depression management
Less severe depression
As shown in the Figure, where an individual meets the criteria for a diagnosis of less severe depression, medication appears to be one of the least cost-effective interventions[19]. NICE therefore advises against the routine prescribing of antidepressants for an episode of less severe depression, unless that is the patient’s preference.
If medication is chosen, selective serotonin re-uptake inhibitors (SSRIs) — such as citalopram, escitalopram, fluoxetine, paroxetine and sertraline — are recommended owing to their relative safety and tolerability[15].
Guided self-help measures are deemed to be the most cost-effective management option in this group[19]. Pharmacists should be aware of any locally available self-help materials that could be offered to the individual. Often, these materials include tips grounded in cognitive behavioural therapy (CBT) and behavioural activation[15,29] (29).
Advice on simple non-pharmacological interventions, such as improved sleep hygiene, exercise, reducing alcohol intake and improving diet, may also be helpful. This information should also be offered to people at risk of developing depression and may be easily incorporated into over-the-counter discussions and structured medication reviews.
For further advice and guidance on this subject, see ‘Encouraging self-care and positive lifestyle changes in patients with depression’[30].
More severe depression
Medication as a standalone treatment for severe depression has been judged by NICE to be less cost-effective than behavioural activation or CBT interventions alone. Antidepressant medication is the fourth most cost-effective intervention, but becomes the most cost-effective intervention when combined with CBT[19]. For more information on CBT and its role in the management of depression, see ‘Further reading‘. Medication recommendations are summarised in Box 3.
Clinicians should consider that some symptoms of depression may impede engagement with therapy (e.g. slow thinking, memory problems or difficulty with attention and concentration). In this scenario, consider starting medication first, with a plan to introduce CBT once the antidepressant has had time to take effect[15].
NICE suggests selecting an SSRI or serotonin and noradrenaline reuptake inhibitor (SNRI) antidepressant, such as venlafaxine or duloxetine, as first-line agents, but emphasises that the individual’s preferences regarding medication — including historical response and concerns about side effects and dependency formation — should also be considered. For example, if they have historically had good response to a TCA (e.g. lofepramine, amitriptyline, imipramine, clomipramine), then that could be considered a first-line medication intervention for the individual, provided they have no contraindications. It is also important to enquire about any overdose history when opting for a TCA as they are extremely cardiotoxic in overdose[15].
Some restrictions are placed on mirtazapine, which NICE recommends be reserved as a further-line treatment option, and vortioxetine, which should only be prescribed if the individual has failed to respond to at least two other antidepressants in the same episode of depression. In both cases, the individual should ideally have been offered psychological intervention in addition to first-line antidepressants and either declined psychological intervention or experienced little improvement from it[15,28].
Box 3: Important considerations when prescribing antidepressants for an episode of more severe depression
- Antidepressants may be more effective when combined with CBT;
- Offer an SSRI, SNRI or, alternatively, an antidepressant that has helped in the past (e.g. TCA);
- Do not routinely prescribe mirtazapine unless first-line antidepressants and psychological intervention have failed to improve symptoms of depression;
- Vortioxetine is a third-line medication option, which should only be prescribed in primary care if the conditions of the relevant NICE technology appraisal are met[6,28].
Chronic depression
A diagnosis of chronic depression or chronic depressive symptoms may be more appropriate where an individual has experienced persistent depressive symptoms over a period of at least two years. The chronicity, rather than the severity, of symptoms is important[15].
When screening for depression in these individuals, it can be helpful for clinicians to begin by asking about the individual’s hobbies or interests rather than directly asking about feelings of sadness, as many people with chronic depressive symptoms do not recognise that they may be depressed[31–33]. For instance, conservative estimates suggest that up to 70% of people with medically unexplained symptoms may be suffering from undiagnosed chronic depression or anxiety, while approximately two thirds of people with a chronic physical health condition are likely to experience symptoms of comorbid mental illness — most commonly depression or anxiety[34,35].
People who present with chronic depressive symptoms should be offered a choice of CBT, medication or combination therapy, as well as targeted rehabilitation support[15].
Where CBT is included in the individual’s treatment plan, the referral to psychological services (known as Improving Access to Psychological Therapies (IAPT), see ‘Further reading‘ for details) should stress the chronicity of the depressive symptoms, so that treatment may be tailored to target chronic depressive symptoms. Providing CBT that specifically targets unhelpful thinking patterns and behaviours associated with chronic depression can help to support rehabilitation of the individual and reduce their sense of social isolation[35].
NICE recommends selecting an SSRI, SNRI or TCA, when medication as a standalone option is selected, or an SSRI or TCA where it is to be combined with CBT[15]. Once a diagnosis of chronic depression has been confirmed, pharmacists can utilise their medicines optimisation skills to ensure that the decision to prescribe medication — if chosen — accounts for the individual’s prescribed medication, over-the-counter remedies if relevant, and underlying physical health conditions. Further guidance on this topic may be found in NICE guidance[34].
Pharmacists also have a role in ensuring management of the underlying physical health condition is optimised. For example, suboptimal ferritin stores or subclinical hypothyroidism may aggravate or contribute to the development of a depressive episode and may reduce the effectiveness of treatment[36–38].
Even when the above strategies are employed, a proportion of individuals will not respond to traditional first-line interventions[39]. The next stage would be to seek advice and support from specialist mental health services. Advice and guidance or e-consult rapid referral pathways may help to avoid delays in accessing further treatment while the individual remains on a waiting list for specialist mental health input[40]. Pharmacists working within primary care networks are encouraged to consult a specialist mental health pharmacist, if available. These specialists can support with antidepressant review and advise on the advantages and disadvantages of further-line medication interventions and any barriers to prescribing locally. This knowledge may save valuable time and enable patients to access further-line treatments sooner[41].
Essential counselling points when starting medication
Pharmacists should ensure that they fully explain the implications of choosing an antidepressant over other options, including the risk of side effects while on treatment and the risk of potentially severe withdrawal symptoms when a dose is missed or when they wish to stop treatment[15,42].
Young people (aged up to 25 years) and those with a history of suicidality should be warned of the increased risk of suicide during the early stages of treatment with antidepressant medication.
Once the patient has confirmed that they wish to proceed with antidepressant medication, antidepressant selection should consider the patient’s past experience of antidepressant treatment and their willingness to tolerate specific adverse effects (e.g. weight gain, sexual dysfunction or sedation)[15,43]. Box 4 highlights some key points that should be covered (in addition to the standard dosing and lifestyle advice) before starting a patient on an antidepressant.
Box 4: Key points to cover when counselling patients on taking antidepressants
- Potential for adverse effects and withdrawal symptoms;
- How to switch or stop treatment safely (contact designated healthcare professional for support with tapering, monitoring for withdrawal symptoms and signs of relapse);
- Recommended duration of treatment (including time to response);
- Details of follow-up (covered below) and how to self-monitor for signs of improvement[6].
Readers are referred to section 1.4 of NICE guidance for a comprehensive explanation of the above counselling points. Links to supporting materials, including patient decision aids and how to withdraw safely (NICE recommends a hyperbolic tapering regimen that is tailored to the individual), are provided in the ‘Further reading‘ section of this article. Additional materials may be available locally from specialist mental health services.
Follow-up of patients prescribed antidepressant medication
As a minimum, patients should be reviewed two to four weeks after starting an antidepressant. At this point, adherence should be evaluated and any underlying issues addressed; for example, side effects or confusion around dosing[20]. The risk of suicide may be slightly increased in the early stages of treatment with an antidepressant; therefore, it is important to proactively enquire about suicidality, as patients will not always be forthcoming about this[44]. Incorporating routine outcome monitoring (e.g. PHQ-9) into each contact point will ensure that suicidality has been assessed and will help with evaluating whether the current prescription is suited to the patient[11,15].
Many pharmacists feel uncertain about how best to support patients following a disclosure of suicidality. Advice specifically aimed at pharmacists, but relevant to all healthcare professionals, is covered in ‘Suicide: how to recognise the warning signs and deal with disclosure‘[45].
Once the patient is in remission, they should be advised to take their antidepressant for a minimum of six months to reduce the risk of relapse. Patients who opt to continue with medication in the long term should be reviewed every six months and given the opportunity to withdraw or reduce medication at each review point. Patients should be informed of the potential for relapse following antidepressant withdrawal[46].
Managing withdrawal symptoms
Box 5 shows the range of symptoms associated with antidepressant withdrawal. Patients should be counselled on the risk of withdrawal symptoms before starting treatment with an antidepressant. Some antidepressants, such as venlafaxine and paroxetine, are associated with more severe withdrawal symptoms, even after missing a single dose, owing to their relatively short half-lives and high potency for the serotonin transporter[6,47]. Patients should be reassured that most people manage to withdraw from antidepressant medication successfully and that a gradual, hyperbolic taper should help to ease withdrawal symptoms[48].
Box 5: Antidepressant withdrawal symptoms
- Unsteadiness, vertigo or dizziness;
- Altered sensations (e.g. electric shock sensations sometimes described as ‘brain zaps’);
- Altered feelings (e.g. irritability, anxiety, low mood, tearfulness, panic attacks, irrational fears, confusion or, very rarely, suicidal thoughts);
- Restlessness or agitation;
- Problems sleeping;
- Sweating;
- Abdominal symptoms (e.g. nausea);
- Palpitations, tiredness, headaches, and aches in joints and muscles[6].
Pharmacists can support patients who are experiencing severe withdrawal effects to taper their antidepressant dose by arranging a switch to liquid formulations to facilitate a slower taper[15,49,50].
Adjunctive medications, polypharmacy and risk of serotonin syndrome
Patients who do not respond to antidepressant monotherapy may express a preference for combination antidepressants or adjunctive medication over psychological intervention. For example, low-dose mirtazapine or trazodone, combined with an SSRI or SNRI, or a medication from a different class, such as a mood stabiliser or an antipsychotic. NICE recommends that specialist input is sought when deciding whether to prescribe such medications, and that lithium or antipsychotics are prescribed within a shared-care agreement[15].
Pharmacists need to be mindful of the potential for drug–drug and patient-drug interactions in such patients. The Maudsley Prescribing Guidelines in Psychiatry provide an at-a-glance overview of key metabolic pathways for centrally-acting drugs, including clinically significant patient–drug interactions, such as if a patient smokes, which antidepressants are affected and to what degree[51]. QT prolongation (an irregular heart rhythm) is frequently observed with lithium and antipsychotics, which are both NICE-endorsed adjunctive treatments for depression. However, they should not be prescribed outside of specialist settings in combination with the SSRIs citalopram or escitalopram, owing to the risk of severe QT prolongation. As the effect is dose-dependent, pharmacists should also be vigilant for any co-prescribed medications which might inhibit their metabolism. A good example is omeprazole, which is commonly prescribed alongside antipsychotics and SSRIs. A baseline electrocardiogram is recommended to exclude any existing cardiac pathology which may further increase the risk of a prolonged QT interval[52].
Pharmacists should be aware of the increased potential for serotonin syndrome in these patients. Serotonin syndrome is a type of neurotoxicity that may occur as a result of serotonin overload (e.g. when a patient takes several pro-serotonergic drugs together, or is prescribed a drug that inhibits the metabolism of their established antidepressant to a significant degree). Pharmacists should extend this vigilance to patients with comorbid physical health issues who, in addition to an antidepressant, may already be prescribed multiple medications affecting serotonergic tone[53]. Examples include:
- Duloxetine or amitriptyline for diabetic neuropathy;
- Tramadol or opioids for chronic pain;
- Tryptans or tryptophan for severe migraine;
- Linezolid for infection;
- Bupropion for smoking cessation (inhibits the metabolism of a variety of antidepressants)[54,55].
Low-grade serotonin syndrome can be easily mistaken for worsening of the underlying condition, particularly if the patient has comorbid anxiety symptoms. Patients may complain of a jittery stomach, sweats and palpitations and seem generally on edge. Look out for signs of neurotoxicity, such as increased muscle tone or random jerking movements, particularly in the lower limbs[53,56].
Every ‘mild’ case of serotonin syndrome has the potential to escalate and become life-threatening. For example, if the dose of a serotonergic agent is increased or if a medication is prescribed that inhibits the metabolism of the patient’s established antidepressant. The timing of the onset of symptoms in relation to recent medication changes should help to confirm any suspicions (signs of serotonin syndrome may develop in a matter of hours)[57].
A good pneumonic to help clinicians recognise the signs and symptoms of serotonin syndrome is ‘SHIVERS’ (see Box 6). Note that not all symptoms may occur. A recent history of serotonergic drug ingestion — including prescribed, over-the-counter and illicit substances — alongside three of the symptoms in Box 6 would be sufficient for a working diagnosis of serotonin syndrome. Suspected cases should be referred for urgent medical attention and the culprit medication(s) withheld[56]. Pharmacists can help to protect patients from this potentially serious adverse treatment effect by ensuring that all patients starting additional serotonergic medication are warned about the risk of serotonin syndrome, are aware of the signs and know to seek prompt medical attention should symptoms develop[58].
Box 6: SHIVERS pneumonic for screening for serotonin syndrome
S hivering – helps distinguish serotonin syndrome from other hyperthermic syndromes
H yperreflexia and myoclonus are common in mild-to-moderate cases; muscular rigidity in severe cases
I ncreased temperature
V ital sign instability — for example, tachycardia, tachypnea, and/or labile blood pressure
E ncephalopathy — notable change in mental state; for example, agitation, delirium, confusion
R estlessness owing to excess serotonergic activity
S weating — distinguishing feature of serotonin syndrome; for example, in neuroleptic malignant, the skin feels hot but dry
Adapted from: MDedge Psychiatry[56]
Summary
Pharmacists in patient-facing roles are well placed to support depression screening programmes and health and wellbeing initiatives, given that they frequently encounter people with elevated risk factors for depression, such as those with chronic physical health conditions and/or polypharmacy[5].
Brief interventional screening offered at key contact points may help to achieve earlier diagnosis. Where depression is suspected, pharmacists can support patients with accessing treatment by signposting to the GP or making a referral to liaison psychiatry services for inpatients[36].
Following a diagnosis of depression, pharmacists can support adherence to antidepressant medication by incorporating shared decision-making into their medicines counselling sessions, and advising on optimal doses for the individual, likely response times and management of side effects[5,23]. Pharmacists should also take note of recommendations in relation to prescribing for less-severe depression. Pharmacists can also help to reduce medication-related harm by advising patients on the potential risks of pharmacological versus non-pharmacological treatments, including educating patients on the signs and symptoms of serotonin syndrome and antidepressant withdrawal syndrome, and ensuring the patient knows how to contact their healthcare professional for support[57] .
Further reading and patient information resources
- Depression screening calculators are available from Medscape Medical Calculators; PHQ-2 (routine screening for depressive symptoms), PHQ-9 (confirmation of depression and its severity);
- The Royal College of Psychiatrists (RCPsych) website provides patient information on topics including: Depression in adults; Sleeping well; Alcohol and depression, Antidepressants; Stopping antidepressants (including the hyperbolic tapering method); and Cognitive behavioural therapy;
- Rethink Mental Illness provides an antidepressants factsheet, which compares different classes of antidepressants and their possible side effects;
- National Institute for Health and Care Excellence (NICE) guidance advises on management of antidepressant withdrawal syndrome;
- MIND provides patient information on depression;
- Depression UK provides patient information on depression;
- Mental Health Foundation has patient information about depression and cognitive behavioural therapy;
- The Samaritans; telephone helpline: 116 123 (freephone), available 24 hours per day;
- SANEline; telephone helpline: 0300 304 7000, open 16:00–22:00 every day;
- NICE provides further information on Improving access to psychological therapies.
- 1Depression. World Health Organization. 2021.https://www.who.int/news-room/fact-sheets/detail/depression (accessed Feb 2023).
- 2Lim GY, Tam WW, Lu Y, et al. Prevalence of Depression in the Community from 30 Countries between 1994 and 2014. Sci Rep. 2018;8. doi:10.1038/s41598-018-21243-x
- 3Pindar J. Depression Statistics UK: 2023. Champion Health. 2023.https://championhealth.co.uk/insights/depression-statistics/ (accessed Feb 2023).
- 4Sobocki P, Jönsson B, Angst J, et al. Cost of depression in Europe. J Ment Health Policy Econ 2006;9:87–98.https://www.ncbi.nlm.nih.gov/pubmed/17007486
- 5The role of pharmacy in mental health and wellbeing: COVID-19 and beyond. Royal Pharmaceutical Society. 2022.https://www.rpharms.com/recognition/all-our-campaigns/policy-a-z/the-role-of-pharmacy-in-mental-health-and-wellbeing (accessed Feb 2023).
- 6Depression in adults: treatment and management. National Institute for Health and Care Excellence. 2022.https://www.nice.org.uk/guidance/ng222 (accessed Feb 2023).
- 7Katon W. The Epidemiology of Depression in Medical Care. Int J Psychiatry Med. 1988;17:93–112. doi:10.2190/xe8w-glcj-kem6-39fh
- 8Baker M, Dorzab J, Winokur G, et al. Depressive disease: Classification and clinical characteristics. Comprehensive Psychiatry. 1971;12:354–65. doi:10.1016/0010-440x(71)90073-3
- 9Diagnostic and statistical manual of mental disorders DSM-5. 5th ed. Washington: : American Psychiatric Association 2013.
- 10Gotlib H, Hammen C, editors. Handbook of Depression. 3rd ed. New York: : Guilford Press 2015.
- 11Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. J Gen Intern Med. 2001;16:606–13. doi:10.1046/j.1525-1497.2001.016009606.x
- 12Stewart D, Madden M, Davies P, et al. Structured medication reviews: origins, implementation, evidence, and prospects. Br J Gen Pract. 2021;71:340–1. doi:10.3399/bjgp21x716465
- 13Palapinyo S, Methaneethorn J, Leelakanok N. Association between polypharmacy and depression: a systematic review and meta‐analysis. J Pharm Pract Res. 2021;51:280–99. doi:10.1002/jppr.1749
- 14Meader N, Mitchell AJ, Chew-Graham C, et al. Case identification of depression in patients with chronic physical health problems: a diagnostic accuracy meta-analysis of 113 studies. Br J Gen Pract. 2011;61:e808–20. doi:10.3399/bjgp11x613151
- 15Recommendations; Depression in adults: treatment and management. National Institute for Health and Care Excellence. 2022.https://www.nice.org.uk/guidance/ng222/chapter/Recommendations#recognition-and-assessment (accessed Feb 2023).
- 16Patient Health Questionnaire‐2. AETC. 2016.https://aidsetc.org/sites/default/files/resources_files/PHQ-2_English.pdf (accessed Feb 2023).
- 17Löwe B, Kroenke K, Gräfe K. Detecting and monitoring depression with a two-item questionnaire (PHQ-2). Journal of Psychosomatic Research. 2005;58:163–71. doi:10.1016/j.jpsychores.2004.09.006
- 18PHQ-9. Snomed. 2023.https://snomedbrowser.com/Codes/Details/758711000000105 (accessed Feb 2023).
- 19Visual summaries; Depression in adults: treatment and management. National Institute for Health and Care Excellence. 2022.https://www.nice.org.uk/guidance/ng222/resources/visual-summaries-11131007005 (accessed Feb 2023).
- 20Depression: Scenario: New or initial management. National Institute for Health and Care Excellence. 2022.https://cks.nice.org.uk/topics/depression/management/initial-management/ (accessed Feb 2023).
- 21Day E, Shah R, Taylor RW, et al. A retrospective examination of care pathways in individuals with treatment-resistant depression. BJPsych open. 2021;7. doi:10.1192/bjo.2021.59
- 22Gosling R, Parry S, Stamou V. Community support groups for men living with depression: barriers and facilitators in access and engagement with services. Home Health Care Services Quarterly. 2021;41:20–39. doi:10.1080/01621424.2021.1984361
- 23Shared decision making. National Institute for Health and Care Excellence. 2023.https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-guidelines/shared-decision-making (accessed Feb 2023).
- 24Schueller SM, Parks AC. Disseminating Self-Help: Positive Psychology Exercises in an Online Trial. J Med Internet Res. 2012;14:e63. doi:10.2196/jmir.1850
- 25van den Bosch RJ, Rombouts RenéP, van Asma MJO. Subjective cognitive dysfunction in schizophrenic and depressed patients. Comprehensive Psychiatry. 1993;34:130–6. doi:10.1016/0010-440x(93)90058-c
- 26Snyder HR, Miyake A, Hankin BL. Advancing understanding of executive function impairments and psychopathology: bridging the gap between clinical and cognitive approaches. Front. Psychol. 2015;6. doi:10.3389/fpsyg.2015.00328
- 27Say R, Murtagh M, Thomson R. Patients’ preference for involvement in medical decision making: A narrative review. Patient Education and Counseling. 2006;60:102–14. doi:10.1016/j.pec.2005.02.003
- 28Vortioxetine for treating major depressive episodes. National Institute for Health and Care Excellence. 2015.https://www.nice.org.uk/guidance/ta367 (accessed Feb 2023).
- 29Huguet A, Rao S, McGrath PJ, et al. A Systematic Review of Cognitive Behavioral Therapy and Behavioral Activation Apps for Depression. PLoS ONE. 2016;11:e0154248. doi:10.1371/journal.pone.0154248
- 30Encouraging self-care and positive lifestyle changes in patients with depression. The Pharmaceutical Journal. 2020. doi:10.1211/pj.2020.20207677
- 31Romano J, Turner J. Chronic pain and depression: does the evidence support a relationship? Psychol Bull 1985;97:18–34.https://www.ncbi.nlm.nih.gov/pubmed/3983297
- 32Hind D, O’Cathain A, Cooper CL, et al. The acceptability of computerised cognitive behavioural therapy for the treatment of depression in people with chronic physical disease: A qualitative study of people with multiple sclerosis. Psychology & Health. 2010;25:699–712. doi:10.1080/08870440902842739
- 33Blazer D, Hughes DC, George LK. The Epidemiology of Depression in an Elderly Community Population. The Gerontologist. 1987;27:281–7. doi:10.1093/geront/27.3.281
- 34Depression in adults with a chronic physical health problem: recognition and management. National Institute for Health and Care Excellence. 2009.https://www.nice.org.uk/guidance/cg91 (accessed Feb 2023).
- 35Long term conditions and medically unexplained symptoms. NHS England. 2023.https://www.england.nhs.uk/mental-health/adults/nhs-talking-therapies/mus/ (accessed Feb 2023).
- 36Depression: What else might it be? National Institute for Health and Care Excellence. 2022.https://cks.nice.org.uk/topics/depression/diagnosis/differential-diagnosis/ (accessed Feb 2023).
- 37Wassef A, Nguyen QD, St-André M. Anaemia and depletion of iron stores as risk factors for postpartum depression: a literature review. Journal of Psychosomatic Obstetrics & Gynecology. 2018;40:19–28. doi:10.1080/0167482x.2018.1427725
- 38Hage MP, Azar ST. The Link between Thyroid Function and Depression. Journal of Thyroid Research. 2012;2012:1–8. doi:10.1155/2012/590648
- 39Little A. Treatment-resistant depression. Am Fam Physician 2009;80:167–72.https://www.ncbi.nlm.nih.gov/pubmed/19621857
- 40Advice and guidance. NHS England. 2023.https://www.england.nhs.uk/elective-care-transformation/best-practice-solutions/advice-and-guidance/ (accessed Feb 2023).
- 41Primary care networks: A briefing for pharmacy teams . NHS England. 2019.https://www.england.nhs.uk/wp-content/uploads/2019/06/pcn-briefing-for-pharmacy-teams.pdf (accessed Feb 2023).
- 42Nederlof M, Cath DC, Stoker LJ, et al. Guidance by physicians and pharmacists during antidepressant therapy: patients’ needs and suggestions for improvement. BMC Psychiatry. 2017;17. doi:10.1186/s12888-017-1522-9
- 43Cartwright C, Gibson K, Read J, et al. Long-term antidepressant use: patient perspectives of benefits and adverse effects. PPA. 2016;Volume 10:1401–7. doi:10.2147/ppa.s110632
- 44Gunnell D, Saperia J, Ashby D. Selective serotonin reuptake inhibitors (SSRIs) and suicide in adults: meta-analysis of drug company data from placebo controlled, randomised controlled trials submitted to the MHRA’s safety review. BMJ. 2005;330:385. doi:10.1136/bmj.330.7488.385
- 45Suicide: how to recognise the warning signs and deal with disclosure. The Pharmaceutical Journal. 2019. doi:10.1211/pj.2019.20205832
- 46Depression: Scenario: Ongoing management. National Institute for Health and Care Excellence. 2022.https://cks.nice.org.uk/topics/depression/management/ongoing-management/ (accessed Feb 2023).
- 47Warner C, Bobo W, Warner C, et al. Antidepressant discontinuation syndrome. Am Fam Physician 2006;74:449–56.https://www.ncbi.nlm.nih.gov/pubmed/16913164
- 48Haddad PM. Antidepressant Discontinuation Syndromes. Drug Safety. 2001;24:183–97. doi:10.2165/00002018-200124030-00003
- 49Framer A. What I have learnt from helping thousands of people taper off antidepressants and other psychotropic medications. Therapeutic Advances in Psychopharmacology. 2021;11:204512532199127. doi:10.1177/2045125321991274
- 50Pearce C. NICE publishes new draft guidance on stopping antidepressants. The Pharmacist. 2021.https://www.thepharmacist.co.uk/news/nice-publishes-new-draft-guidance-on-stopping-antidepressants/ (accessed Feb 2023).
- 51Taylor D, Barnes T, Young A. The Maudsley Prescribing Guidelines in Psychiatry. John Wiley & Sons 2021.
- 52Citalopram and escitalopram: QT interval prolongation. Medicines and Healthcare products Regulatory Agency. 2014.https://www.gov.uk/drug-safety-update/citalopram-and-escitalopram-qt-interval-prolongation (accessed Feb 2023).
- 53Isbister GK, Buckley NA, Whyte IM. Serotonin toxicity: a practical approach to diagnosis and treatment. Medical Journal of Australia. 2007;187:361–5. doi:10.5694/j.1326-5377.2007.tb01282.x
- 54Serotonin syndrome. Mayo Clinic. 2022.https://www.mayoclinic.org/diseases-conditions/serotonin-syndrome/symptoms-causes/syc-20354758 (accessed Feb 2023).
- 55Quinn DK, Stern TA. Linezolid and Serotonin Syndrome. Prim. Care Companion J. Clin. Psychiatry. 2009;11:353–6. doi:10.4088/pcc.09r00853
- 56Christensen R. Get serotonin syndrome down cold with SHIVERS. MDedge. 2006.https://www.mdedge.com/psychiatry/article/62032/get-serotonin-syndrome-down-cold-shivers (accessed Feb 2023).
- 57Simon L, Keenaghan M. Serotonin Syndrome. National Library of Medicine. 2022.http://www.ncbi.nlm.nih.gov/books/NBK482377/ (accessed Feb 2023).
- 58Singh H, Saadabadi A. Sertraline. National Library of Medicine. 2022.https://www.ncbi.nlm.nih.gov/books/NBK547689 (accessed Feb 2023).


