www.dnaillustrations.com
After reading this article, you should be able to:
- Understand the rationale for nasal preparations for local and systemic drug delivery;
- Describe suitable methods for the administration of nasal preparations;
- Manage local side effects of topical nasal preparations.
Intranasal administration offers convenient access to the nasal cavity and surrounding paranasal sinuses and has been used for local drug delivery to treat common minor ailments, such as sinusitis and allergic rhinitis[1]. Pharmacists and pharmacy teams regularly recommend over-the-counter preparations — including saline drops and irrigations, and decongestant and steroid sprays — to relieve symptoms of the common cold, influenza, hayfever and rhinosinusitis. Therefore, they should be familiar with these and should feel confident to advise patients on their correct administration.
This article outlines the use of nasal preparations for local or systemic drug delivery; the appropriate administration of nasal drops, sprays and rinses; and how to manage local side effects.
Available formulations
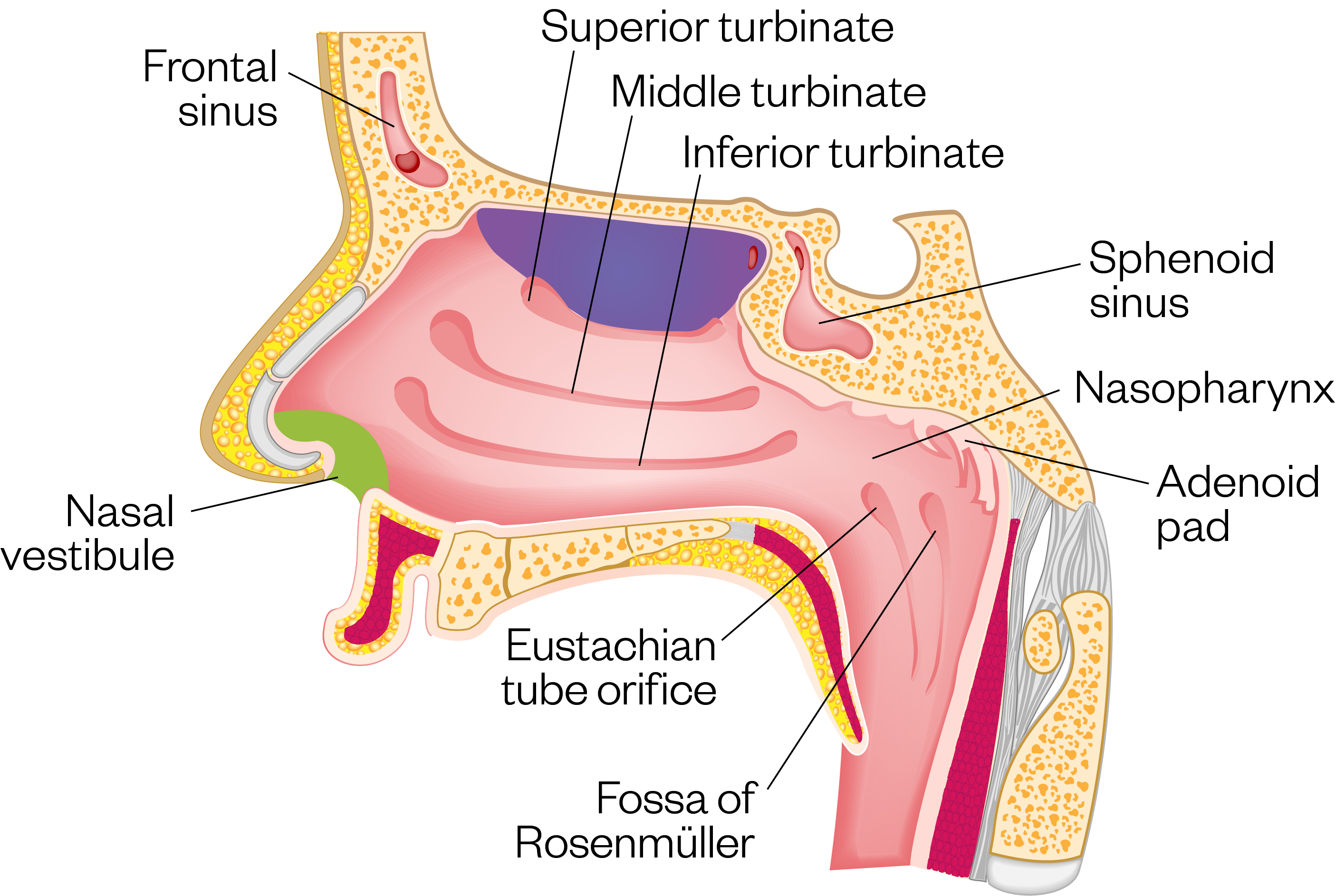
Intranasal formulations (e.g. sprays, drops and ointments) aim to deliver drugs to the nasal cavity while limiting further travel along the respiratory tract. The nasal cavity itself can be broadly split into three sections (see Figure 1): the vestibule (at the entrance of the nose), the atrium, and the respiratory regions (see Figure 2)[2]. The respiratory zone — which has folds called turbinates that filter and humidify inhaled air for the lungs — has good vascularity and surface area, making it a useful site for systemic drug delivery. The olfactory zone, located at the roof of the nasal cavity close to the central nervous system (CNS), has been suggested to aid penetration across the blood brain barrier[1].
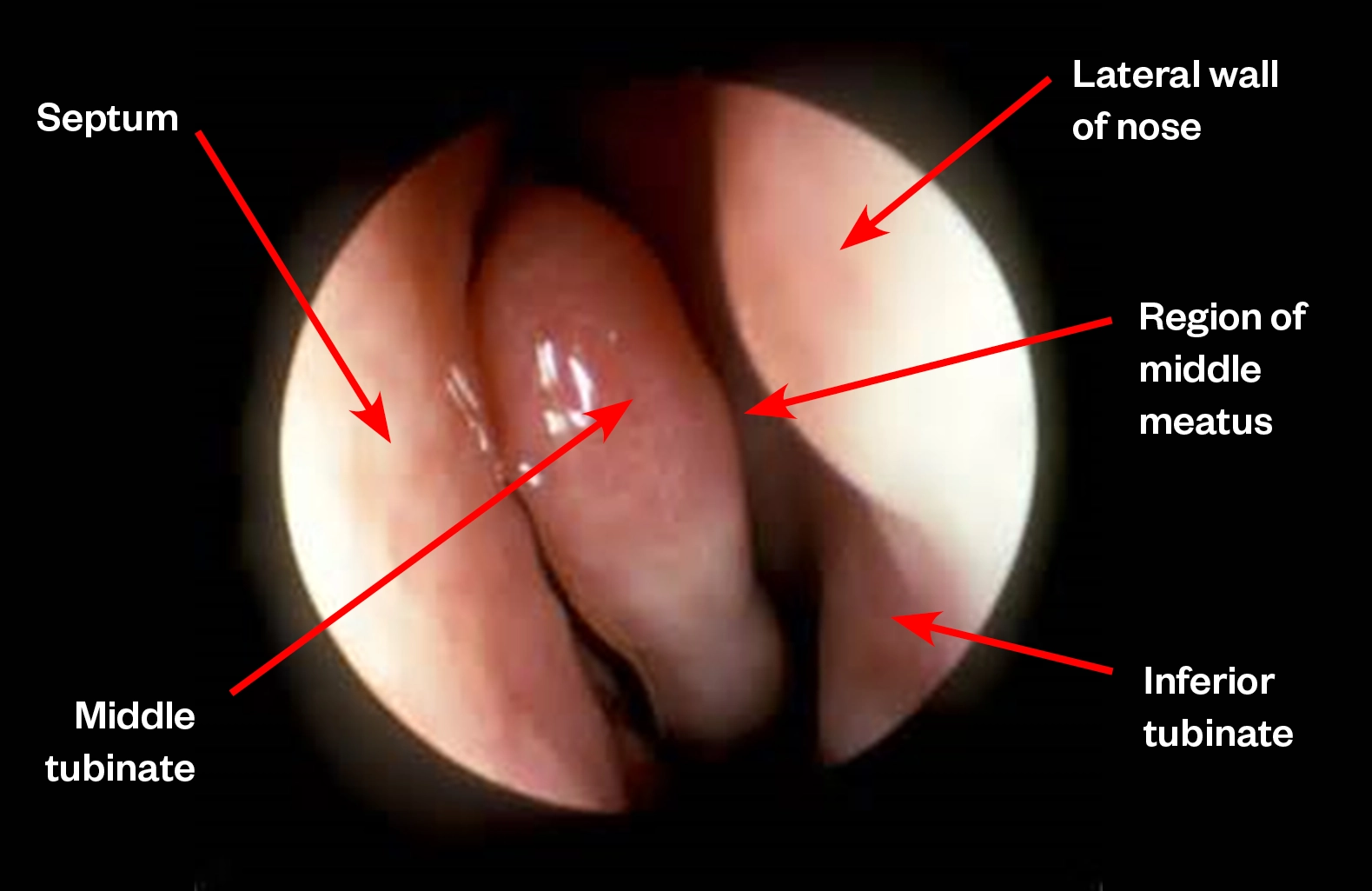
Nasal drops are used for the temporary relief of congestion in the nose caused by colds, sinusitis, hayfever and allergies. Decongestants (e.g. xylometazoline), which are available as drops as well as a spray, cause the blood vessels to constrict, reducing the swelling and associated congestion. When administered correctly, drops effectively deliver steroids (e.g. fluticasone) to the middle meatus and are generally more effective than steroid sprays for the management of nasal polyps[3]. Some patients with rhinosinusitis dislike using nasal drops owing to some of the advised head positions for administration that can exacerbate symptoms[3].
Nasal sprays are more commonly used to deliver locally acting medications (e.g. antihistamines and steroids) and can provide some humidification and wider distribution throughout the nasal cavity. Aqueous preparations have reduced microbial stability, and the addition of preservatives may result in nasal irritation and allergy[1]. The fixed volume per actuation allows better control over drug dosing, which can be an issue with multi-dose nasal drops[4].
Nasal ointments and creams offer prolonged retention within the nasal cavity and are mainly used for the treatment of bacterial nasal cavity infections and nose bleeds[5]. The higher viscosity of these preparations is thought to reduce the effect of mucociliary clearance of the medication, prolonging the duration of action. Pharmacists and their teams should be aware that one preparation contains arachis oil (Naseptin; Alliance Pharmaceuticals) and, as a result, they need to ensure that the patient is not allergic to peanuts[6]. Some patients may experience local irritation (e.g. burning, stinging, itching and pain) from components of the ointments and creams, and may be allergic to the constituents including the antibiotic(s). Systemic side effects include severe stomach pain, diarrhoea, itching and/or skin rash. Petroleum jelly is frequently used to reduce irritation resulting from dryness, crust formation and to prevent nose bleeds[7]. Usually this clears to the back of the nose and then swallowed; however, there is the potential for it to enter the lungs and cause potentially serious inflammation known as lipoid pneumonia[8].
Nasal irrigation involves flushing the nasal cavity with isotonic or hypertonic saline solution. It is commonly used in conjunction with other medical treatments, to prevent and treat rhinosinusitis, allergic rhinitis and upper respiratory tract infections, especially in children[9]. Although the exact mechanism is unknown, it has been speculated that the solution draws out water from the surrounding tissue, while removing antigens and inflammatory mediators to improve mucociliary clearance[9,10].
Efficacy of nasal route of administration
The advantages of intranasal administration include the ability to avoid first pass hepatic and gastric metabolism, while offering a rapid onset of action comparable with intravenous formulations[2]. It also allows the delivery of larger macromolecules (e.g. desmopressin and the influenza vaccine)[11,12]. Being less painful and invasive, intranasal influenza vaccines are favoured in paediatric patients and are routinely used in clinical practice[4].
Limitations
Various factors can affect drug absorption and therefore the efficacy of the intranasal route. The drug physiochemical profile itself needs to be considered. Drugs of low molecular weight, higher lipophilicity and those in the unionised state have optimal permeability across the nasal mucosa[2]. This is illustrated with intranasal formulations of drugs such as fentanyl, which has high lipophilicity and low molecular weight and has been found to have almost 100% bioavailability[13]. This has enabled fentanyl to be useful in the management of breakthrough pain in cancer care[2]. The formulation is important since particle size of the drug influences the site of deposition and the viscosity impacts on the drug absorption[1,12].
A limitation of intranasal formulations are that drugs are required to have good water solubility to allow the full dose to be present in 0.2–0.3mL per nostril[4,12]. This can significantly limit the dosage of poorly water-soluble drugs making them less viable options[13], although administration of partial doses split between each nostril can help optimise absorption[4]. Conditions that affect mucociliary clearance, such as cystic fibrosis and diabetes mellitus, and nasal blood flow, such as the concurrent use of vasoconstrictors, can act as potential barriers to intranasal absorption[2].
Despite the benefits of intranasal delivery, patients on long-term nasal oxygen or non-invasive ventilation may be less suitable candidates. Patients with recent radiation to the head and neck or those who are at risk of bleeding, may be advised to avoid intranasal therapy[14].
Counselling patients on administration
The remainder of this article will provide advice on how pharmacists and pharmacy teams can advise patients on correct administration technique, to avoid common mistakes.
Before the use of either a nasal spray or drops, patients should be advised to gently blow their nose, then wash their hands and shake the nasal spray or drops before removing the cap. This will ensure the potential for infection is minimised and effective delivery of the intranasal formulation[15]. If patients experience an unpleasant tasteafter using a nasal preparation, they should be advised to drink water or a flavoured beverage, as this may help. This, along with nasal soreness, bleeding and persistence of symptoms may be indicative of incorrect technique, and patients should be reminded of the correct techniques, as outlined below[16,17].
Nasal sprays
Pharmacists and pharmacy teams should advise the patient:
- To keep the head upright, insert nozzle tip into one nostril keeping the other nostril open;
- Hold the bottle with the index and middle finger at the top and the thumb at the bottom;
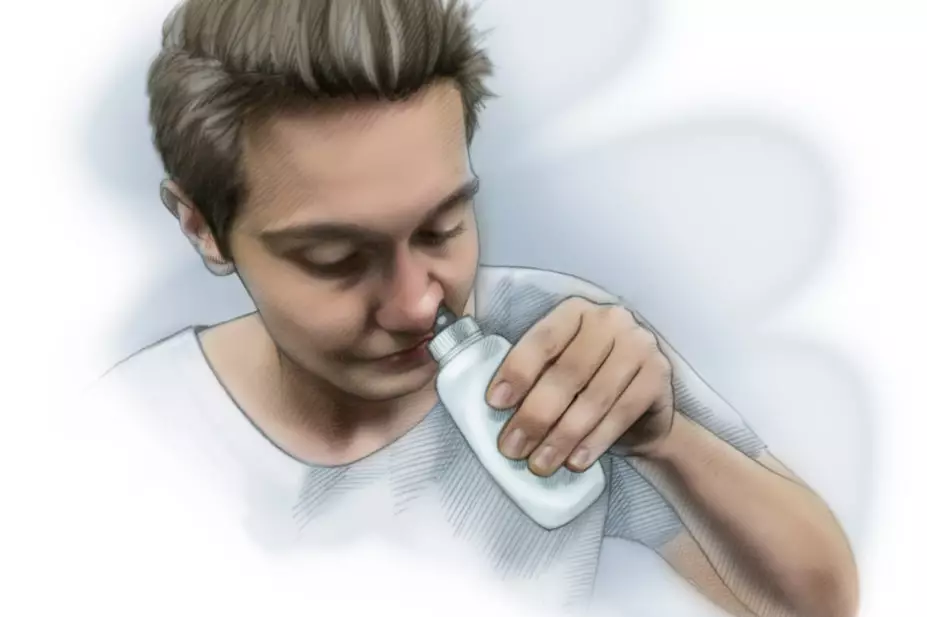
- To use their right hand to spray the left nostril and vice versa, so that the spray is directed away from the septum (see Image 1). Holding the spray with the same hand as the nostril (e.g. right hand for right nostril) increases the risk of nasal irritation and bleeding since this directs the spray towards the nasal septum[18];
- That sprays should be administered while they slowly breathe in through their nose. After removing the nozzle, they should breathe out through the mouth;
- To repeat for the other nostril before cleaning the nozzle and replacing the cap[19–21].
Other considerations
Overuse of some nasal sprays, such as decongestants, should be avoided as this can lead to rebound congestion (rhinitis medicomentosa), where tissue swelling worsens each time the nasal spray is stopped[22]. This creates a cycle of continued use.

www.dnaillustrations.com
Nasal drops
Pharmacists and pharmacy teams should advise the patient:
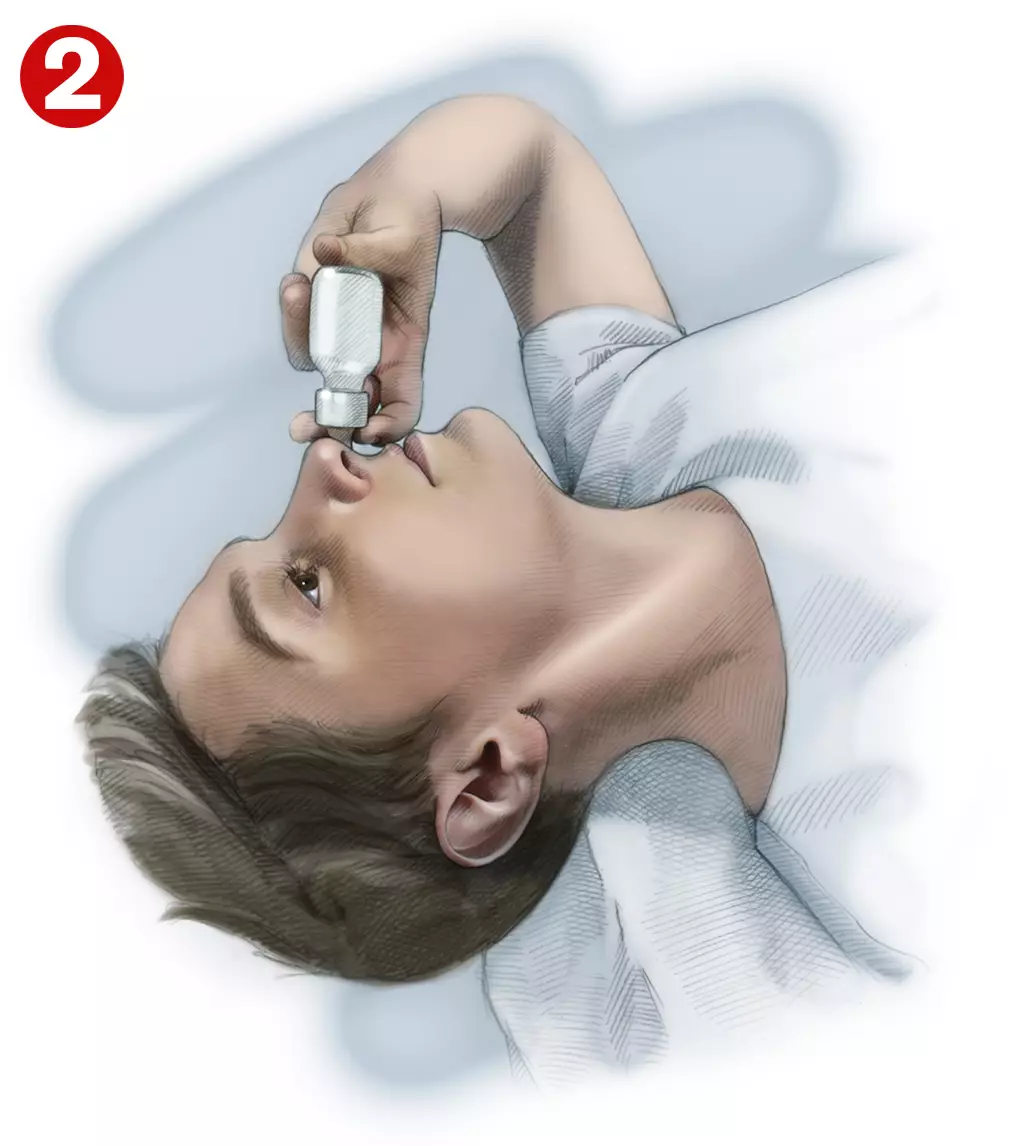
- To lie on their back with the head just off the bed and tilted so the chin is highest point of the head. Patients should breathe normally through the mouth while instilling the prescribed number of drops into each nostril (see Image 2). Administration of drops while sitting or standing with the head tilted backwards causes more solution to pass down the back of the throat[18];
- To hold this position for two minutes after drop instillation. This enables the steroid to reach the region where polyps arise and reduce inflammation where the sinuses open into the nasal cavity;
- If multi-dose, manufacturers recommend that the dropper should be cleaned by wiping the nozzle with a clean tissue before replacing the cap[23]. However, Tan et al. found cross-contamination could be reduced by sterilisation with boiling water, ethanol wipes and microwaving[16].
It is important to understand that nasal sprays and drops are not always effective at reaching the sinuses, even post-surgery, and this has lead some rhinologists to advise combining nasal drops with saline irrigation[16]. It is the larger volume that improves penetration to the sinuses[24].
www.dnaillustrations.com
Nasal ointment
Pharmacists and pharmacy teams should advise the patient:
- To squeeze a pea-sized amount of ointment onto the tip of the little finger and apply to the inside surface at the front of each nostril (see Image 3). If using a single-use tube, insert half the contents of the tube into each nostril (see Image 4);
- Gently press the nostrils together and massage to help spread the ointment throughout the nose[25] (see Image 5). Not squeezing nostrils after administration prevents adequate distribution and absorption of the ointment;
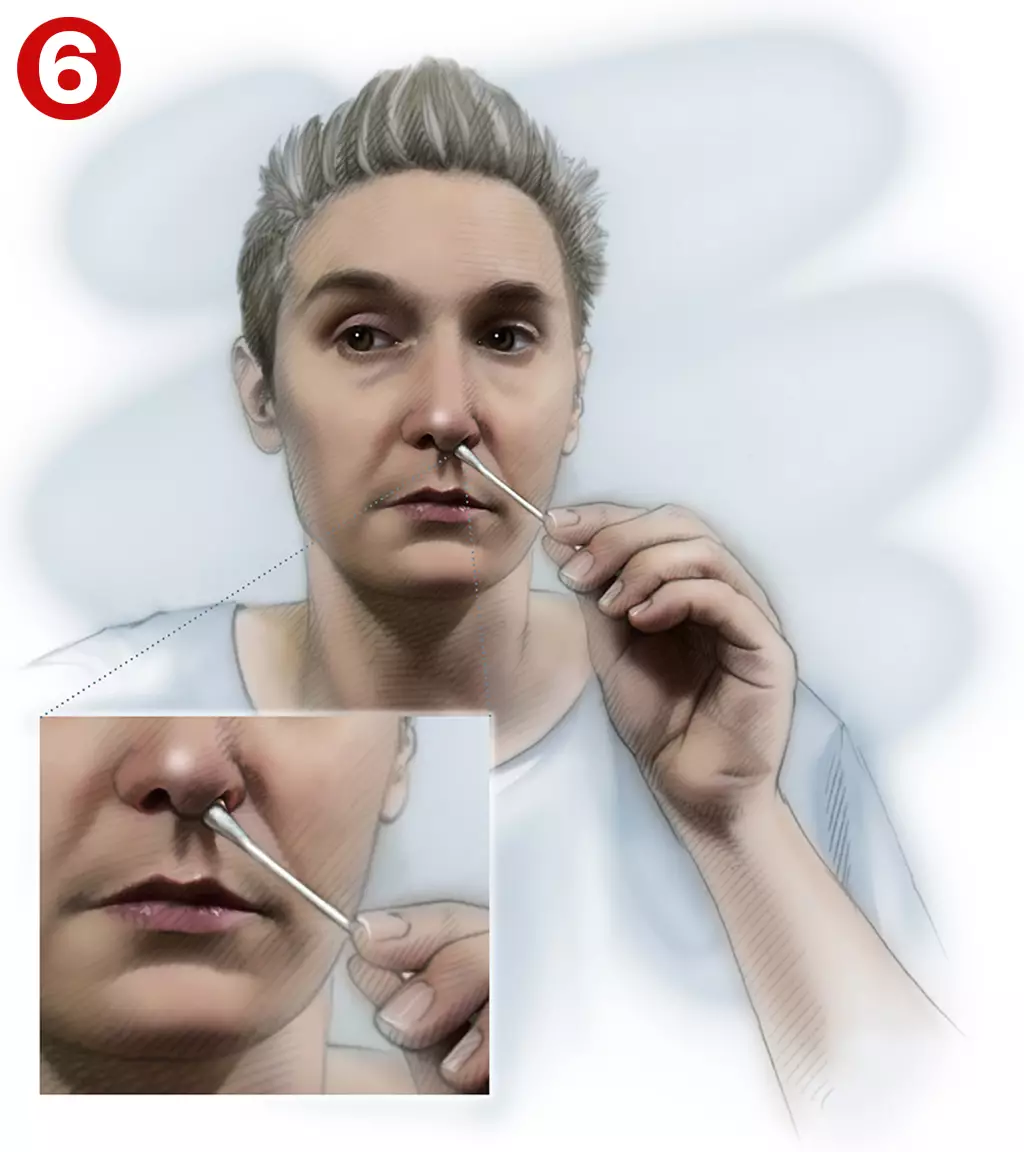
A swab, such as a cotton bud, may be used for application to infants or patients who are very ill[25] (see Image 6).

www.dnaillustrations.com

www.dnaillustrations.com

www.dnaillustrations.com
www.dnaillustrations.com
Nasal irrigation
Pharmacists and pharmacy teams should advise the patient:
- That if they are also using intranasal medication, this should be used after nasal irrigation. This is because the medication will have better distribution and efficacy[15];
- To stand in front of a basin, tilt the head away from the bottle, and squirt the solution into each nostril, aiming the stream toward the back of the head, not the top (see Image 7). The solution may flow into one nostril and out the other[15] (see Image 8);
- To avoid breathing through the nose while preforming irrigation, as this can introduce water into the ear canal potentially leading to infection[9];
- Sometimes a burning sensation in the nasal cavity occurs particularly if a non-buffered hyper or hypotonic irrigation solution is used; however, most patients seem to adapt to this[15].
- Where possible, the use of pre-mixed irrigation solutions and sterile water should be used to reduce the risk of infection[9].

www.dnaillustrations.com

www.dnaillustrations.com
Summary
It is important that nasal preparations are administered in an appropriate manner to ensure the effective delivery of the medication. Pharmacists and pharmacy teams should educate and reassure patients on their use and management of any unpleasant symptoms or side effects, should these occur.
In addition, the importance of using saline and steroid preparations regularly while limiting the use of decongestant sprays to three to five days should be emphasised when recommending or dispensing these products.
This article has been reviewed by the expert authors to ensure it is relevant and up to date, following its original publication in October 2021.
- 1Keller L-A, Merkel O, Popp A. Intranasal drug delivery: opportunities and toxicologic challenges during drug development. Drug Deliv and Transl Res Published Online First: 25 January 2021. doi:10.1007/s13346-020-00891-5
- 2Grassin-Delyle S, Buenestado A, Naline E, et al. Intranasal drug delivery: An efficient and non-invasive route for systemic administration. Pharmacology & Therapeutics 2012;134:366–79. doi:10.1016/j.pharmthera.2012.03.003
- 3Djupesland PG. Nasal drug delivery devices: characteristics and performance in a clinical perspective—a review. Drug Deliv and Transl Res 2012;3:42–62. doi:10.1007/s13346-012-0108-9
- 4Clinical Practice Guideline: Intranasal Medication Administration. Journal of Emergency Nursing 2018;44:5.e1-5.e43. doi:10.1016/j.jen.2017.11.003
- 5Béquignon E, Teissier N, Gauthier A, et al. Emergency Department care of childhood epistaxis. Emerg Med J 2016;34:543–8. doi:10.1136/emermed-2015-205528
- 6Naseptin nasal cream. Electronic medicines compendium. 2017.https://www.medicines.org.uk/EMC/medicine/2503/SPC/Naseptin+Nasal+Cream (accessed Oct 2021).
- 7Burton M, Dorée C. Interventions for recurrent idiopathic epistaxis (nosebleeds) in children. Cochrane Database Syst Rev 2004;:CD004461. doi:10.1002/14651858.CD004461.pub2
- 8Brown A, Slocum P, Putthoff S, et al. Exogenous lipoid pneumonia due to nasal application of petroleum jelly. Chest 1994;105:968–9. doi:10.1378/chest.105.3.968
- 9Principi N, Esposito S. Nasal Irrigation: An Imprecisely Defined Medical Procedure. IJERPH 2017;14:516. doi:10.3390/ijerph14050516
- 10Neilmed® Sinus RinseTM . London: Guy’s and St Thomas’ NHS Foundation Trust. 2021.https://www.guysandstthomas.nhs.uk/resources/patient-information/pharmacy/neilmed-sinus-rinse.pdf (accessed Oct 2021).
- 11Gao M, Shen X, Mao S. Factors influencing drug deposition in the nasal cavity upon delivery via nasal sprays. J Pharm Investig 2020;50:251–9. doi:10.1007/s40005-020-00482-z
- 12Marx D, Williams G, Birkhoff M. Intranasal Drug Administration — An Attractive Delivery Route for Some Drugs. In: Drug Discovery and Development – From Molecules to Medicine. InTech 2015. doi:10.5772/59468
- 13Pires A, Fortuna A, Alves G, et al. Intranasal Drug Delivery: How, Why and What for? J Pharm Pharm Sci 2009;12:288. doi:10.18433/j3nc79
- 14Intranasal medication administration. Winnipeg Regional Health Authority. 2021.https://professionals.wrha.mb.ca/old/extranet/eipt/files/EIPT-055.pdf (accessed Oct 2021).
- 15How to perform nasal irrigation. UpToDate. 2021.https://www.uptodate.com/contents/image?imageKey=ALLRG%2F71059&topicKey=PC%2F83012&source=see_link (accessed Oct 2021).
- 16Tan NC-W, Drilling AJ, Jardeleza C, et al. Is nasal steroid spray bottle contamination a potential issue in chronic rhinosinusitis? J Laryngol Otol 2013;128:S28–33. doi:10.1017/s0022215113001229
- 17Advice on using nose drops. Royal Berkshire NHS Foundation Trust. 2019.https://www.royalberkshire.nhs.uk/patient-information-leaflets/head%20and%20neck%20ent%20using%20nose%20drops (accessed Oct 2021).
- 18Using nose drops . Kernow Clinical Commissioning Group. 2021.https://rms.kernowccg.nhs.uk/content/files/Using%20nose%20drops%20and%20sprays.pdf (accessed Oct 2021).
- 19Benninger MS, Hadley JA, Osguthorpe JD, et al. Techniques of Intranasal Steroid Use. Otolaryngol Head Neck Surg 2004;130:5–24. doi:10.1016/j.otohns.2003.10.007
- 20Menditto E, Costa E, Midão L, et al. Adherence to treatment in allergic rhinitis using mobile technology. The MASK Study. Clin Exp Allergy 2019;49:442–60. doi:10.1111/cea.13333
- 21Scadding GK, Kariyawasam HH, Scadding G, et al. BSACI guideline for the diagnosis and management of allergic and non-allergic rhinitis (Revised Edition 2017; First edition 2007). Clin Exp Allergy 2017;47:856–89. doi:10.1111/cea.12953
- 22Treating chronic sinusitis. Institute for Quality and Efficiency in Health Care. 2012.https://www.ncbi.nlm.nih.gov/books/NBK279484/ (accessed Oct 2021).
- 23Fluticasone nasal spray and drops. NHS. 2020.https://www.nhs.uk/medicines/fluticasone-nasal-spray-and-drops/ (accessed Oct 2021).
- 24Merkus P, Ebbens F, Muller B, et al. The ‘best method’ of topical nasal drug delivery: comparison of seven techniques. Rhinology 2006;44:102–7.https://www.ncbi.nlm.nih.gov/pubmed/16792167
- 25Bactroban nasal ointment. NPS MedicineWise. 2018.https://www.nps.org.au/medicine-finder/bactroban-nasal-ointment (accessed Oct 2021).


