TEK IMAGE / SCIENCE PHOTO LIBRARY
After reading this article, you should be able to:
- Describe hyponatraemia and appreciate the need for its judicious management;
- Understand the role of the extracellular cation sodium and be familiar with the reference range;
- List the investigations needed to confirm the cause of hyponatraemia, including serum osmolality, glucose, cortisol, thyroid function tests, urine osmolality and urine sodium;
- Apply knowledge from clinical cases to establish whether a clinical presentation of hyponatraemia is acute or chronic and describe relevant treatment plans;
- Identify the common causes of hyponatraemia.
Hyponatraemia occurs when an individual has a serum sodium concentration of <135mmol/L. Although it can present in any healthcare setting, it is the most common disorder of body fluid/electrolyte balance encountered in the acute setting and is frequently classified as a medical emergency in the emergency department or critical care[1]. Hyponatraemia is present in around 15–20% of emergency admissions to hospital and has high morbidity and mortality rates (see Table 1)[1,2].
Despite its prevalence, management of hyponatraemia is frequently sub-optimal[3]. Results from a prospective, case–control study of 208 patients showed that patients with hyponatraemia had an overall mortality rate of 27%, compared with 9% for patients without hyponatraemia[1]. Given that hyponatraemia presents across a range of specialties, there is considerable variation in its management, which may explain the difference in mortality. Some of the challenges of diagnosis include assessing fluid status or ‘balance’ and the chronicity of hyponatraemia at presentation[2].
This article provides a comprehensive, evidence-based review of clinical presentation and treatment pathways for hyponatraemia. Clinical cases are used to further illustrate common presentations.
Signs and symptoms
Symptoms of hyponatraemia vary from mild and non-specific to severe and life-threatening. Patients are more likely to show symptoms of hyponatraemia if there is an acute drop in sodium concentration. Conversely, patients that are chronically hyponatraemic are usually asymptomatic[4].
Hyponatraemia can present as chronic or acute. Chronic cases tend to be either asymptomatic or have mild symptoms, such as nausea, malaise, fatigue, dizziness and muscle cramps. Acute cases tend to have more severe symptoms, such as vomiting, cardio-respiratory failure, abnormal/deep somnolence, convulsions/seizures and coma (Glasgow coma scale <8)[2].
The role of sodium
Sodium is the principal extracellular cation and its main purpose is to maintain plasma osmolality[3]. It plays a fundamental role in the maintenance of total body water and the regulation of blood pressure. In addition, it is essential for the generation of action potentials in nervous and cardiac tissue[3].
The intracellular sodium concentration is approximately 12mmol/L, whereas extracellular sodium is between 133–146 mmol/L, meaning that serum sodium status must be assessed with fluid volume status[3]. Sodium absorption occurs in the distal small bowel and colon. The balance of sodium and water is finely maintained by the kidneys and sodium filtered by the glomeruli is re-absorbed in a proportion ranging from 0.5% to 10.0%, depending on the action of different hormones and neurotransmitters, including aldosterone and vasopressin[5].
Hyponatraemia is therefore primarily a ‘water-balance disorder’. It is commonly associated with a disturbance in the hormone that regulates water balance, vasopressin (also known as antidiuretic hormone)[5].
Case 1: Syndrome of inappropriate antidiuretic hormone secretion
MK is a female, aged 35 years, and works in a nursery. She was referred to the emergency department with suspected sepsis. MK had a five-day history of worsening cough, elevated temperature, difficulty speaking in full sentences and low arterial blood pressure. In the emergency department, MK tested positive for influenza A and her chest X-ray reported consolidation at both bases. Blood tests revealed elevated C-reactive protein (CRP) and neutrophilia. Arterial blood gas tests revealed a type 1 respiratory failure.
A provisional diagnosis was made of influenza A, secondary bacterial infection and sepsis. MK was admitted for supportive therapy including oseltamivir, IV azithromycin and co-amoxiclav, supplemental oxygen via a venturi face mask and crystalloid fluid replacement. After admission, she had a serum sodium of 122mmol/L. MK appeared mildly dehydrated because of her infections. Paired plasma and urinary osmolalities were undertaken. A diagnosis was made by virtue of a low plasma osmolality of 240 mOsm/kg and raised urine osmolality of 357 mOsm/kg. A provisional diagnosis of syndrome of inappropriate antidiuretic hormone secretion (SIADH) secondary to a lower respiratory tract infection (LRTI) was proposed (pneumonia and LRTIs are a known cause of SIADH). Treatment was judicious management of her fluid balance. In the short term, her fluid balance was normalised with regular assessment of extracellular sodium (as the infection had made MKK intravascularly deplete). Once balance was restored, her fluids were restricted to support the slow correction of serum sodium by a rate of no greater than 8mmol/day[6].
Where SIADH is chronic, solute loss may exceed water retention and may not be managed with fluid restriction alone — drug therapy with sodium supplementation may be required[7]. A loop diuretic may be an option to increase urine volume and decrease urinary sodium concentration[7]. Traditionally, the tetracycline antimicrobial demeclocycline has been used in SIADH but, in recent years, the introduction of specific vasopressin receptor antagonists, such as tolvaptan, means that persistent SIADH can be managed by a specific pharmacotherapy[7].
Deficiencies in serum sodium do not usually occur because of low dietary sodium, even in diets very low in salt[3]. An example of dietary cause of hyponatraemia is ‘beer potomania’, so called because it is caused by an excessive intake of alcohol, particularly beer, together with a poor diet.
Conversely, diets that are high in sodium do not usually cause hypernatremia. This is primarily because of the ability of the kidney to eliminate large quantities of sodium. However, high sodium intake is associated with increases in extracellular fluid and, subsequently, arterial blood pressure[3].
Receptors in the hypothalamus detect changes in cell stretch resulting from systemic, effective osmolality. A reduction in cell stretch, detected by baroreceptors in the left atrium, carotid artery and aortic arch, is transmitted to the vagal nerve, and leads to release of vasopressin from the pituitary gland. Vasopressin enhances water reabsorption in the distal tubules of the nephron, resulting in concentrated urine and fluid retention[5].
Other agents that regulate sodium include aldosterone (which exchanges potassium for sodium in the distal tubule), angiotensin 2 (by sodium re-absorption in the proximal tubule), and atrial natriuretic peptide (which suppresses the renin–angiotensin–aldosterone system (RAAS), and thus sodium excretion from the collecting duct)[5].
Case 2: Malnutrition and dilutional hyponatraemia
SJ is a male, aged 50 years, with a previous medical history of low mood, inflammatory bowel disease and alcohol dependence. SJ had a six-week history of feeling unwell and reported a significant reduction in taste and smell. This resulted in a reduction in appetite and food intake over the six-week period. He continued to drink water and four to five cans of cider per day. In the emergency department, he had episodes of vomiting and was increasingly lethargic. His medication history was oral thiamine 100mg three times daily.
A blood test revealed severe hyponatraemia, with sodium levels of 110 mmol/L. Despite the lethargy, SJ was oriented in time and date and had a normal mini-mental state examination. His blood test results are displayed in Table 2.
Serum and urinary sodium and osmolality were compared (see Table 3).
If a person’s calorie intake decreases markedly over several weeks then total body stores of sodium are depleted, resulting in a decreased ability of the body to maintain homeostasis[8,9]. The ability of the cells to eliminate sodium in exchange for potassium is compromised, leading to increased intracellular sodium and decreased plasma sodium concentrations (although total body sodium can remain in the normal range). In the case of SJ, this was exacerbated by excessive intake of fluids with low sodium content (cider and water)[9].
SJ was approximately six weeks into a low nutritional and high fluid intake phase. In this phase, the kidney is attempting to conserve sodium hence the exceptionally low sodium level in the urine.
A diagnosis of chronic hyponatraemia secondary to poor oral intake with excessive alcohol/fluid intake was proposed, because of patient history and the combination of hyponatraemia, low serum and urine osmolality. SJ was admitted and a treatment plan to cautiously rehydrate and increase serum sodium levels by no more than 6–8mmol/L over 24 hours was introduced. Serum sodium tests were undertaken twice daily. SJ was transferred to critical care for management of hyponatraemia.
SIADH: syndrome of inappropriate antidiuretic hormone secretion; EABV: effective arterial blood volume
Drug causes of hyponatraemia
A variety of causes of hyponatraemia have been described above, but it is important to be aware of the iatrogenic causes listed below. Pharmacists are ideally placed to intervene when patients present with hyponatraemia and are on one or more offending agents.
Medicines that can cause hyponatraemia do so via a range of mechanisms[10,11]. Some drugs affect water and sodium homeostasis (e.g. diuretics):
- Thiazides;
- Indapamide;
- Amiloride, spironolactone;
- Loop diuretics.
Thiazide diuretics are particularly associated with hyponatraemia and should be stopped in most circumstances. One instance where thiazide-like diuretics may be continued is in extreme fluid overload, when they are added to loop diuretics to work synergistically to induce diuresis. Usually, this is when a loop diuretic is insufficient as monotherapy.
Some medicines increase hypothalamic production of antidiuretic hormone:
- Antidepressants (e.g. tricyclic antidepressants, selective serotonin reuptake inhibitors, monoamine-oxidase inhibitors);
- Antipsychotics (e.g phenothiazines, butyrophenones, haloperidol);
- Some anti-seizure medication (e.g. carbamazepine, oxcarbazepine, sodium valproate);
- Anticancer drugs (e.g. vinca alkaloids, platinum compounds, alkylating agents).
Other medicines can potentiate the effects of antidiuretic hormone:
- Antiseizure (e.g. carbamazepine, lamotrigine);
- Non-steroidal anti-inflammatory drugs;
- Anticancer drugs (e.g. alkylating medicines).
Carbamazepine and oxcarbazepine are particular culprit medicines for causing hyponatraemia. They increase ADH, which in turns leads to increased absorption of water in the kidneys. A retrospective review of 1,782 patients admitted with epilepsy found a prevalence of hyponatraemia in 26% of patients taking carbamazepine and 46% taking oxcarbazepine. One risk factor is high serum levels of the medicine; therefore, it is worth considering therapeutic drug monitoring in this cohort, as the degree of hyponatraemia is dose dependent[12]. An alternative should be used if lower doses are not suitable/tolerated, especially where the indications is epilepsy/seizures.
Diagnosis
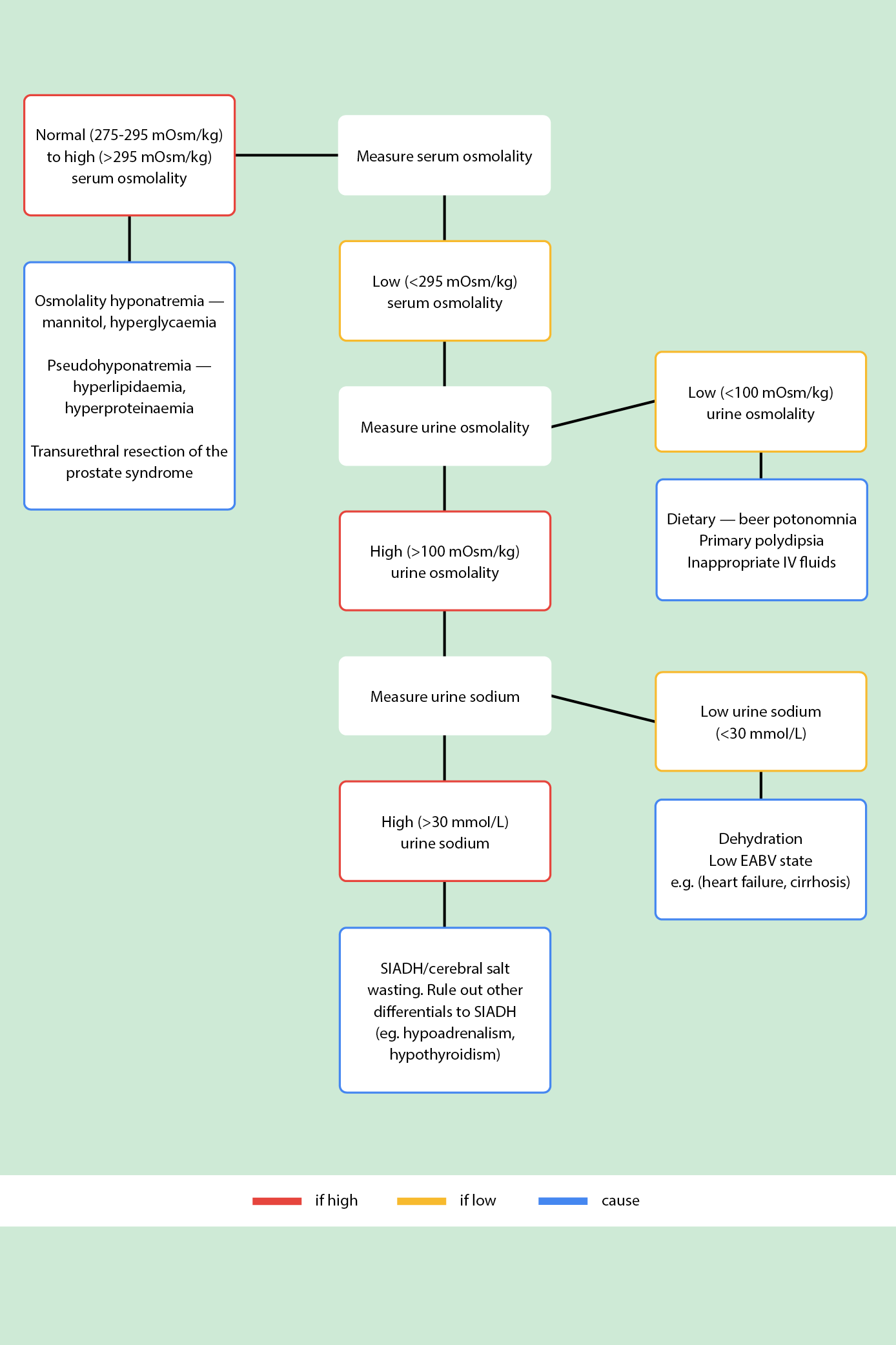
National guidelines and consensus statements recommend assessing fluid status of patients within the pathway of diagnosing the cause of hyponatraemia[7]. In practice, this is difficult to achieve unless the patient is either dehydrated or excessively fluid overloaded[2]. However, systematic methods of determining the cause of hyponatraemia using laboratory tests — including serum osmolality, urine osmolality and urine sodium — should facilitate better management of hyponatraemia. In clinical practice, these tests should be undertaken simultaneously, and the results interpreted alongside clinical judgement of the patient’s presentation.
1. Serum osmolality
Low serum osmolality (<275 mOsm/kg) is true hyponatraemia. If a suspected hyponatraemia case presents with normal (275–295 mOsm/kg) to high (>295 mOsm/kg) serum osmolality, there might be an excess of additional (non-sodium) osmotically active agents in the blood stream (e.g. lipids or glucose). This is commonly referred to as a pseudohyponatraemia. Lipid profile, glucose level and serum protein should be ascertained to effectively manage the underlying cause (e.g. statins for hypercholesterolaemia and insulin for hyperglycaemia)[2].
Transurethral resection of the prostate syndrome is another iatrogenic hyponatraemia, which is iso/hyperosmolar, but not pseudohyponatraemia. This type of hyponatraemia occurs during endoscopic, urological or gynaecological procedures that require continuous irrigation of hypotonic fluids and results in systemic absorption of the irrigation fluid[13].
2. Urine osmolality
Urine osmolality helps determine whether the kidneys have retained their ability to dilute and concentrate urine. Urine osmolality is principally used in the diagnosis of SIADH. The appropriate response to serum hypotonicity is for the kidneys to produce large volumes of dilute urine to restore serum tonicity.
Low urine osmolality (<100mOsm/kg) is a normal response to hyponatraemia. It has several causes:
- Dietary: often referred to as ‘beer potomania’ or the ‘tea and toast’ diet. Hyponatraemia can occur because of a poor diet; for instance, in alcohol dependency or in patients whose diets are deficient in sodium. In mild hyponatraemia, these patients can be treated by improving nutrition (e.g. by involving a dietician). In profound hyponatraemia (<115–120 mmol/L), hospital admission and judicious replacement is required;
- Primary polydipsia: excess of water intake. The most dramatic cases of primary polydipsia are seen in psychiatric patients, particularly those with acute psychosis secondary to schizophrenia[2];
- Inappropriate IV fluids: patients admitted to hospital who receive large amounts of IV infusions, such as crystalloids, can develop hyponatraemia. Table 4 lists the sodium content and osmolarity of common IV fluids. Dextrose saline (sodium chloride 0.18%, glucose 4%) is frequently used as a maintenance fluid and contains only 31mmol/L of sodium. This is lower than 0.9% sodium chloride, in which the sodium content is closer to the reference range for plasma sodium (154mmol/L of sodium). Excessive fluid resuscitation with low sodium fluids can result in dilutional hyponatraemia[14].
Table 4 shows the composition of commonly used crystalloid fluids[15].
High urine osmolality (>100 mOsm/kg) is urine that is inappropriately concentrated (impaired water excretion). Thyroid function tests and random cortisol should be checked to exclude other causes, such as hypothyroidism and/or hypoadrenalism. In the absence of dehydration, a high urine osmolality is a strong correlator to SIADH.
3. Urine sodium
Urine sodium distinguishes between low circulating blood volume (urine sodium <30 mmol/L) and SIADH (urine sodium >30mmol/L). Low urine sodium is a normal RAAS response to hypovolamia (low body fluid volume) but can be an abnormal overreaction to an ‘apparent’ low-volume state.
Conditions that have a lower effective arterial blood volume (EABV) include heart failure, cirrhosis of the liver and kidney failure. Decreases in circulating blood volume and renal under-perfusion activate the RAAS and increase water retention, resulting in peripheral and pulmonary oedema[2]. These conditions are managed by sodium and water restriction, and commonly require diuretics[2].
In the absence of conditions that present with low EABV, the patient may be truly hypovolemic. If so, treatment is the administration of sodium chloride (0.9%) and monitoring of serum sodium. Causes of blood volume depletion include vomiting, diarrhoea, burns, excessive diuresis, and salt-losing nephropathies[16]. A patient with SIADH will continue to manifest an inappropriate antidiuretic hormone (ADH) response and continue to eliminate sodium, so will not have a sufficient increase in serum sodium when administered fluids with high sodium concentration.
High urine sodium (>30 mmol/L) is consistent with SIADH. A diagnosis of SIADH is typically made by ruling out other diagnoses — such as cardiac, liver, renal and thyroid disease — and diuretic therapy.
Case 3: Cerebral salt wasting
KS is a woman, aged 28 years, who presented to the emergency department by ambulance after collapsing at her evening exercise class. She had suddenly complained of a ‘shotgun’ headache during the class, vomited and had a seizure.
On arrival, she was taken immediately for a computed tomography (CT) angiogram, after which a provisional diagnosis of subarachnoid haemorrhage secondary to an atrio-venous malformation was proposed. KS was referred to neurosurgery and underwent emergency neurosurgery to coil the atrio-venous malformation. Afterwards, KS was admitted to the neurological intensive care unit (NICU).
In the NICU, she developed a polyuria of 250–500mL/h. Serum and urinary sodium and osmolality were checked hourly. Serial blood tests reported a severe drop in serum sodium to 115mmol/L (from 139mmol/L). As the timelines for hyponatraemia were unknown, this was nominally deemed acute hyponatraemia.
Cerebral salt wasting is a cause of hyponatraemia in the setting of central nervous system disease, especially sub-arachnoid haemorrhage[11]. There is also elevated urinary sodium and hypovolemia present[11]. Management is normal fluid replacement and sodium supplementation, as opposed to treatment in SIADH, in which the patient is fluid restricted. Cerebral salt wasting tends to resolve within weeks or months. Even in this setting where hyponatremia is common, osmotic demyelination syndrome (ODS) can occur, and cautious replacement of sodium is still required (8mmol in 24 hour) to prevent osmotic demyelination syndrome (ODS)[17].
Cases and appropriate treatment
There is a paucity of clinical trial data on hyponatraemia, with the exception of recent randomised trials of vaptans[18]. In an ongoing randomised, controlled trial (RCT), participants have been allocated to target correction of sodium versus standard of care. This RCT assesses combined risk of death or rehospitalisation[19]. The findings of this trial could help advise how prudent sodium correction should be in the hospitalised patient.
Risks of rapid correction of hyponatraemia
When serum sodium is rapidly corrected, severe central nervous system symptoms often present (e.g. confusion, tremor and loss of consciousness). This is as a result of brain oedema and a subsequent increase in intracranial pressure[20].
The compensatory mechanisms that the brain has in chronic hyponatraemia include reduction in electrolytes and organic osmolytes (small solutes used by cells to maintain cell volume), which preserves cell volume by preventing the influx of water. This alleviates brain swelling and preserves neuronal function. This adaption process is relatively slow, taking up to 48 hours to occur. Therefore, hyponatraemia is defined as acute (<48 hours) and chronic (>48 hours)[20].
In a patient with chronic hyponatraemia, rapid correction of serum sodium levels leads to a significant osmotic gradient across the blood–brain barrier. In the setting of sudden hypertonicity, because of excessively rapid correction of sodium, astrocytes (star-shaped glial cells in the brain and spinal cord) are unable to rapidly reaccumulate the depleted osmolytes. In severe cases, this can result in osmotic demyelination syndrome (ODS), which is where dehydration of brain cells leads to destruction of the myelin sheath around nerves. Symptoms of ODS often take two to three days to appear and include difficulty speaking, dysphagia, paralysis, and impaired co-ordination[17]. ODS is much more likely to occur if sodium is rapidly corrected. Other underlying risk factors which increase the risk of ODS are alcohol dependence, liver disease, use of thiazides and antidepressants[2].
It is recommended that where the chronicity of hyponatraemia is not established (or considered to be possibly longer than 48 hours, as in case 3), the rate of sodium correction should be slow.
Traditionally, it was advised that the rate of sodium replacement was no greater than 0.5 mmol/L/h; this approximates to 12 mmol/24 hours. This has been recently revised to a maximum increase of 8 mmol/24 hours, owing to reports of ODS in patients with a sodium increase of 8–12 mmol/L/day[6].
Hyponatraemia in patients with neurological symptoms can be corrected rapidly on presentation (e.g. 5 mmol/L within the first hour with hypertonic saline and the remainder over the next 24 hours), but this must be completed with expert input, from a consultant endocrinologist or neurologist, for example[6]. Except in the emergency department, the use of hypertonic saline to rapidly correct hyponatraemia should be done in a level 2 monitored environment, with judicious checking of serum sodium as frequently as hourly[7].
Best practice for pharmacy professionals
- A broad understanding of the role of sodium is essential for pharmacy professionals;
- Knowledge of pathology tests in hyponatraemia is recommended;
- Clinical pharmacy professionals should be aware of daily sodium requirements and relevant sodium concentrations of crystalloid fluids;
- Appreciation and understanding of the dangers of rapid correction of serum sodium concentration is necessary for best practice;
- Understanding and differentiation of most common causes of hyponatraemia, including syndrome of inappropriate anti-diuretic hormone secretion (SIADH) and cerebral salt wasting is recommended for pharmacy professionals in the acute setting.
Practical tips for pharmacists
- Hyponatraemia can be broadly defined as either acute (<48 hours duration) or chronic (>48 hours duration). Be aware that whether hyponatraemia is acute or chronic will change its management with regard to how quickly sodium can be corrected;
- Check for other causes of hyponatraemia, such as cortisol deficiency and hypothyroidism, particularly before making a diagnosis of SIADH. Check glucose and lipid profiles to exclude pseudohyponatraemia;
- Consider whether there are iatrogenic causes, such as thiazide diuretics, carbamazepine and selective serotonin reuptake inhibitors (SSRIs). Discontinue these where possible; in high-risk conditions, such as a history of seizures, an alternative should be used and may require advice from a neurologist. SSRIs require slow tapering because of the risk of withdrawal symptoms. In severe hyponatraemia, sudden discontinuation may be required and the patient counselled on withdrawal symptoms.
- Acute severe/symptomatic hyponatraemia should be corrected with hypertonic saline, ideally in a level 2 care environment. The rate of sodium replacement should not exceed 8mmol per day.
- Pharmacological therapy can be considered in patients with SIADH where hyponatraemia is persistent and fluid restriction is ineffective, impractical or not tolerated. Tolvaptan is licensed and targets the underlying pathology of SIADH but should not be combined with therapies such as fluid restriction or hypertonic saline, because of the risk of too-rapid correction of serum sodium[21].
Declarations of interest and funding
Renita D’Souza, Jennifer Thomson and James Allen report no conflicts of interest.
Cathrine A McKenzie receives funding from the National Institute for Health and Care Research (NIHR) Applied Research Collaborative (ARC) Wessex. The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
- This article was amended on 27 March 2023 to correct an error in Table 4
- 1Gill G, Huda B, Boyd A, et al. Characteristics and mortality of severe hyponatraemia ? a hospital-based study. Clin Endocrinol. 2006;65:246–9. doi:10.1111/j.1365-2265.2006.02583.x
- 2Spasovski G, Vanholder R, Allolio B, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. European Journal of Endocrinology. 2014;170:G1–47. doi:10.1530/eje-13-1020
- 3Strazzullo P, Leclercq C. Sodium. Advances in Nutrition. 2014;5:188–90. doi:10.3945/an.113.005215
- 4Biswas M, Davies JS. Hyponatraemia in clinical practice. Postgraduate Medical Journal. 2007;83:373–8. doi:10.1136/pgmj.2006.056515
- 5Cuzzo B, Padala S, Lappin S. Physiology, Vasopressin. Statpearls 2022. https://www.ncbi.nlm.nih.gov/books/NBK526069/ (accessed Mar 2023).
- 6Hannon M, Thompson C. Neurosurgical Hyponatremia. JCM. 2014;3:1084–104. doi:10.3390/jcm3041084
- 7Grant P, Ayuk J, Bouloux P, et al. The diagnosis and management of inpatient hyponatraemia and SIADH. Eur J Clin Invest. 2015;45:888–94. doi:10.1111/eci.12465
- 8Nielsen AJ, Mose FH. Hyponatremia and Acute Kidney Injury as a Consequence of Malnutrition: A Case Report. Case Rep Clin Nutr. 2021;4:1–6. doi:10.1159/000513712
- 9Boulter PR, Hoffman RS, Arky RA. Pattern of sodium excretion accompanying starvation. Metabolism. 1973;22:675–83. doi:10.1016/0026-0495(73)90239-4
- 10Allen J, Newland-Jones P. How to manage adults with hyponatraemia. The Pharmaceutical Journal. 2012.https://pharmaceutical-journal.com/article/ld/how-to-manage-adults-with-hyponatraemia (accessed Mar 2023).
- 11Tenny S, Thorell W. Cerebral Salt Wasting Syndrome. Statpearls 2022. https://www.ncbi.nlm.nih.gov/books/NBK534855/#:~:text=Cerebral%20salt%20wasting%20is%20characterized,of%20antidiuretic%20hormone%20(SIADH). (accessed Mar 2023).
- 12Berghuis B, van der Palen J, de Haan G-J, et al. Carbamazepine- and oxcarbazepine-induced hyponatremia in people with epilepsy. Epilepsia. 2017;58:1227–33. doi:10.1111/epi.13777
- 13Baiu I, Kang M, Weiser TG. Acute severe iatrogenic hyponatremia. Trauma Surg Acute Care Open. 2019;4:e000388. doi:10.1136/tsaco-2019-000388
- 14Adrogué HJ, Tucker BM, Madias NE. Diagnosis and Management of Hyponatremia. JAMA. 2022;328:280. doi:10.1001/jama.2022.11176
- 15Intravenous fluid therapy in adults in hospital Clinical guideline [CG174]. National Institute for Health and Care Excellence. 2013.https://www.nice.org.uk/guidance/cg174/resources/cg174-intravenous-fluid-therapy-in-adults-in-hospital-composition-of-commonly-used-crystalloids-table-2 (accessed Mar 2023).
- 16Sahay M, Sahay R. Hyponatremia: A practical approach. Indian J Endocr Metab. 2014;18:760. doi:10.4103/2230-8210.141320
- 17Singh TD, Fugate JE, Rabinstein AA. Central pontine and extrapontine myelinolysis: a systematic review. Eur J Neurol. 2014;21:1443–50. doi:10.1111/ene.12571
- 18Chen S, Zhao J-J, Tong N-W, et al. Randomized, double blinded, placebo-controlled trial to evaluate the efficacy and safety of tolvaptan in Chinese patients with hyponatremia caused by SIADH. The Journal of Clinical Pharmacology. 2014;54:1362–7. doi:10.1002/jcph.342
- 19Refardt J, Pelouto A, Potasso L, et al. Hyponatremia Intervention Trial (HIT): Study Protocol of a Randomized, Controlled, Parallel-Group Trial With Blinded Outcome Assessment. Front. Med. 2021;8. doi:10.3389/fmed.2021.729545
- 20Wijayabandara M, Appuhamy S, Weerathunga P, et al. Effective treatment of osmotic demyelination syndrome with plasmapheresis: a case report and review of the literature. J Med Case Reports. 2021;15. doi:10.1186/s13256-020-02573-9
- 21Samsca 30 mg tablets. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/7974/smpc (accessed Mar 2023).
5 comments
You must be logged in to post a comment.



I think there is an error in the table where Sodium Chloride 0.18% and Glucose 4% is repeated twice, do the authors mean Sodium Chloride 0.45% with Glucose 5% but osmolarity would be different than quoted in the table. Also, in the paragraph under the table "Thyroid Function rests". I assume 'tests' would be more appropriate.
Is there a role of hypertonic saline? Especially when considering the acuity of hyponatraemia i.e. <48hours vs chronic.
Strange that spironolactone is not mentioned as one of the diuretics that hyponatraemia particularly the higher doses used to treat ascites
Hi Kevin, thank you for bringing this to our attention. This was an error and has now been corrected.
Best wishes,
Sophie, senior subeditor
Hi Kevin,
Thank you for pointing out the typo's in the table, and elsewhere. This has been kindly addressed by Sophie and the team at the PJ.
With regard to your question about use of hypertonic saline, I think it was touched on in the article, albeit not in detail.
I would say it does not have a role in routine management of hyponatremia, especially in chronic hyponatremia (>48hrs) because of the risk of ODS. I would say never in the absence of close monitoring - hence why we have advocated it be done at least in a level 2 environment where those facilities are available.
However in severe hyponatremia presenting with neurological symptoms, typically acute rather than chronic (although it can be a rapid drop in pre-existing chronic hyponatremia), can be treated if there are adequate monitoring requirements to avoid the risk of over-correction.
In these subset of patients, we would give infusions of 150mL 3% hypertonic saline (or equivalent) over 20 mins aiming for a 5mmol/L increase ideally within the first hour, and then not more than 10mmol/L in the first day.
This use (i.e. the use of hypertonic saline) is advocated by the European guidelines - found here (https://eje.bioscientifica.com/view/journals/eje/170/3/G1.xml)
Hope that was the answer you were looking for.
Thanks,
Renita
Thanks Renita, great article of a complicated subject. Case studies were great. Interesting NEJM released some RCT data from Canada regarding ODS is rare and sodium may not play as big a role as we think https://evidence.nejm.org/doi/full/10.1056/EVIDoa2200215. However, in practice I have seen the devastating effects of ODS and hypertonic saline always makes me worry....