John Walsh / Science Photo Library
After reading this article, you should be able to:
- Recognise the contribution of medication to male factor infertility;
- Be aware of drugs that are potentially teratogenic and advise patients to seek advice from their specialist team prior to attempting conception;
- Understand the rationale and evidence base of medical treatments for male factor infertility;
- Appreciate the duration and potential side effects of pharmacological treatment of male factor infertility.
Infertility is the inability to conceive after one year of regular unprotected intercourse. It is estimated that infertility affects one in seven heterosexual couples in the UK, with 30% of these cases being attributed to the man[1]. In a quarter of cases, no identifiable cause is found and male factor subfertility is classed as idiopathic[2].
Sperm production is hormonally driven and precisely controlled. In normal physiology, sperm is produced when gonadotrophin releasing hormone (GnRH), a neuropeptide secreted from specialised neurones of the hypothalamus, stimulates follicle stimulating hormone (FSH) and luteinizing hormone (LH) secretion from the anterior pituitary gland. LH in turn stimulates Leydig cells in the testes to synthesise testosterone while FSH acts on Sertoli cells in the testes to promote spermatogenesis. Final maturation of the spermatozoa is dependent on intra-testicular testosterone[3].
Disrupted testicular maturation or hormonal imbalances affecting any stage of spermatogenesis can result in infertility. Male factor subfertility is often medically or surgically treatable and can be connected to the use of certain drugs or medicines.
This article provides an overview of the pathophysiology of male infertility, as well the diagnosis and assessment and treatment options, highlighting opportunities for pharmacist involvement.
Causes of male infertility
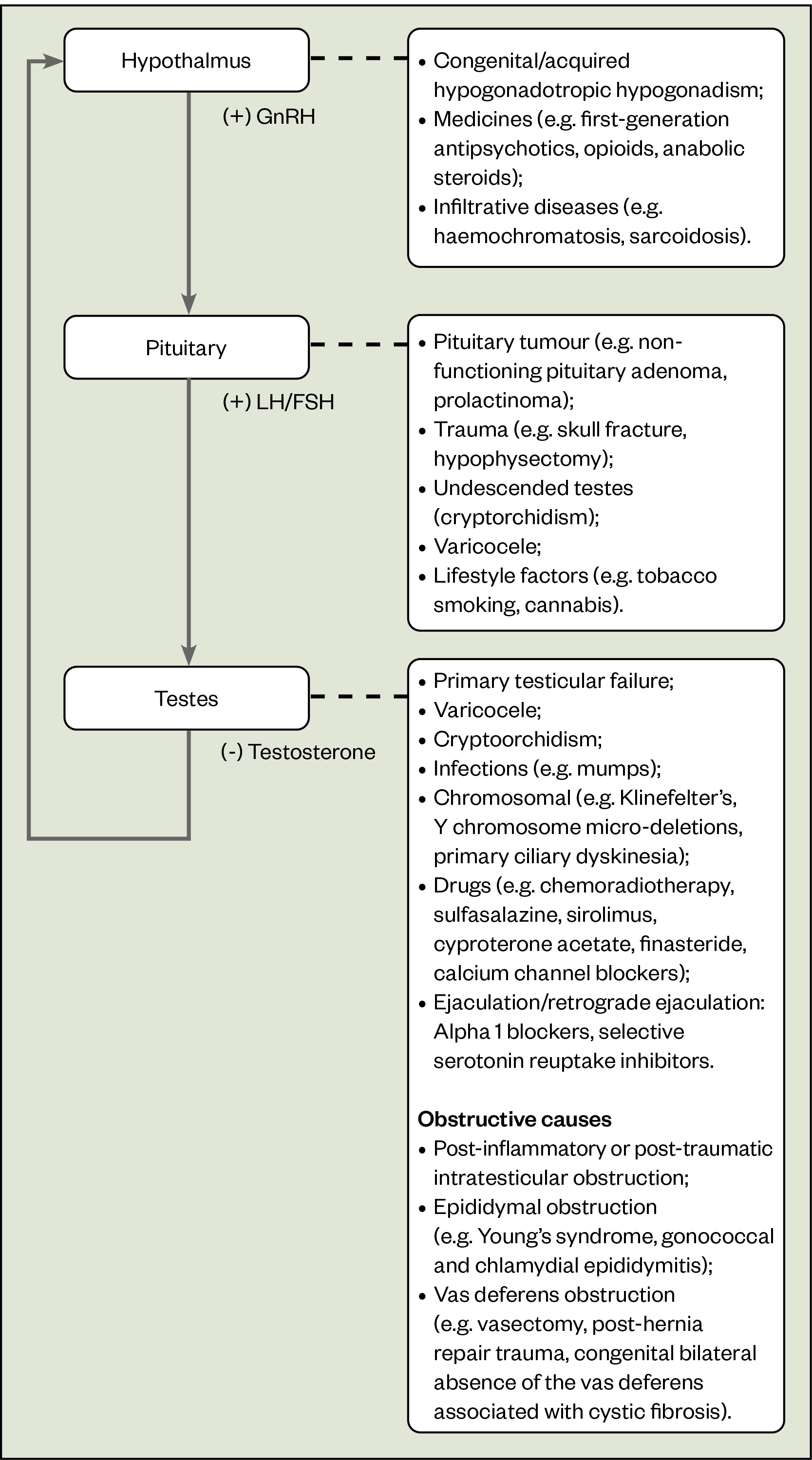
Causes of male infertility may be classified into four broad groups (see Figure):
- Impaired sexual function;
- Primary testicular defects;
- Endocrinopathies that reduce spermatogenesis;
- Defects in sperm transportation[4].
Idiopathic primary testicular dysfunction remains the most common cause of male factor infertility[5].
Impaired sexual function
Preserved erectile function and ejaculation are prerequisites for penetrative intercourse. Erectile function results from the combination of physical and sexual stimulation. It is mediated through the parasympathetic system and its effect on smooth muscle. Neurological disorders, such as multiple sclerosis, and trauma can therefore result in loss of erections. Cardiovascular disorders affecting small vessels can also interfere with erections. Erectile dysfunction is common in men with cardiovascular disease and often precedes a diagnosis of cardiovascular disease[6]. Pharmacists can discuss this with patients and encourage lifestyle modification, including smoking cessation, weight loss and exercise, and encourage screening for conditions such as diabetes and hypertension.
Drugs are also often implicated: verapamil, beta blockers, antidepressants, 5 alpha reductase inhibitors, and certain recreational drugs — such as cocaine — can all cause erectile dysfunction[7,8].
Primary testicular defects
Primary testicular defects refer to inherent testicular problems resulting in decreased sperm and/or testosterone production. These can be inherited, acquired or idiopathic. Inherited defects may result from microdeletions in the AZF region of the Y chromosome or caused by an abnormal karyotype, such as Klinefelter syndrome (also known as ’47, XXY’), typically secondary to one extra X-chromosome.
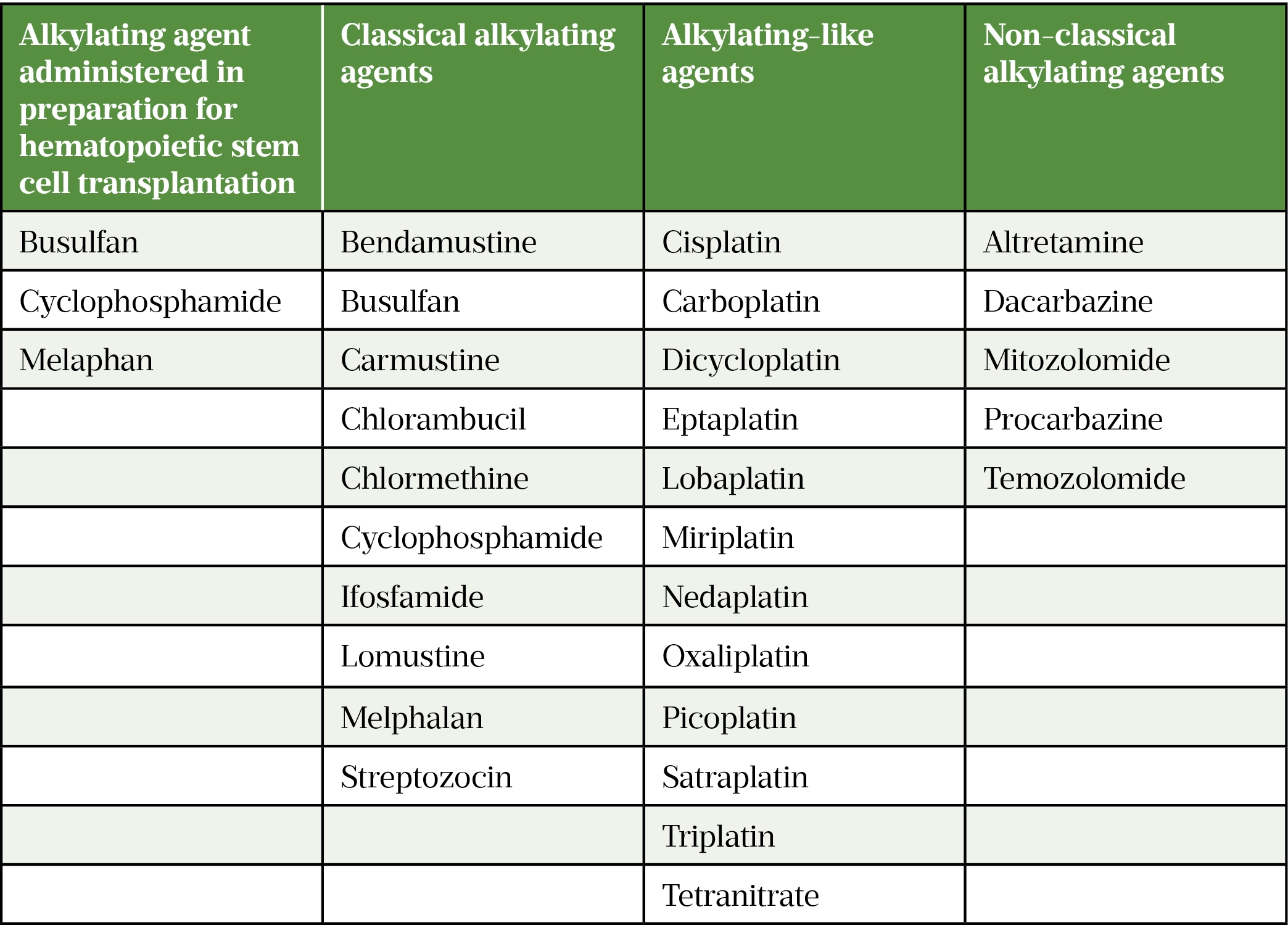
Acquired defects may be traumatic owing to testicular lesions or iatrogenic following chemotherapy, pelvic or testicular surgery/irradiation. Oncologic therapies that are at high risk of inducing infertility include alkylating agents administered in preparation for hematopoietic stem cell transplantation and chemotherapy regimens including ‘classical’ alkylating agents, ‘alkylating-like’ agents and ‘non-classical’ alkylating agents (see Table 1)[5].
Crypto-orchidism and infection (mumps orchitis) may also contribute to an abnormal semen analysis[4].
Endocrinopathies
Male hypogonadism, a condition in which there is low testosterone, can affect spermatogenesis. Hypogonadism can be classed as pre-testicular or testicular (see Figure).
Pre-testicular refers to conditions affecting the hypothalamus or pituitary gland that result in low GnRH, LH or FSH, and a subsequent reduction in testosterone. This is commonly known as hypogonadotropic (low FSH and LH) hypogonadism (low testosterone)[7].
Testicular causes are conditions where there is a problem in the testes resulting in insufficient testosterone production. Low testosterone feeds back to the hypothalamus and the pituitary gland stimulates to produce GnRH and LH/FSH respectively in an effort to then stimulate testosterone production from the testes. This is known as hypergonadotropic (high FSH and LH) hypogonadism (low testosterone). Making the distinction between hypogonadotropic and hypergonadotropic hypogonadism is essential because this determines treatment strategies[4].
Hypogonadotropic hypogonadism (HH) can be congenital, acquired or functional. The latter refers to hypogonadism that occurs in the absence of intrinsic structural hypothalamo-pituitary-gonadal (HPT) axis pathology and the absence of specific pathologic conditions suppressing the HPT axis[8].
Congenital causes of hypogonadotropic hypogonadism
HH can arise from the hypothalamus (deficient production, secretion or action of GnRH) or from the pituitary gland (deficient FSH and LH secretion). GnRH-releasing neurones develop from the olfactory bulb, which also gives rise to the nasal epithelium, and normally migrate from the nasal region to the hypothalamus in the first few weeks of embryonic development. Failure of development of the olfactory bulb results in failure of generation and migration of GnRH neurones and subsequent HH with no olfactory function. This is defined as Kallman syndrome[9]. In cases of normal olfactory bulb development but impaired GnRH neuronal migration or GnRH neuronal function, olfactory function remains unaffected (normosmotic HH)[6].
Acquired causes of hypogonadotropic hypogonadism
There are several acquired causes of HH, including pituitary tumours, infiltrative diseases, infections, trauma, vascular complications and drugs (opioids, corticosteroids, anabolic steroids)[4].
Prolactinomas (tumours of the pituitary gland) are caused by lactotroph adenomas and can cause hypogonadism by both direct inhibitory effect of prolactin on LH and FSH secretion, but also by tumour effect on the pituitary gland itself[10]. Drugs causing hyperprolactinaemia, an elevated prolactin level in the blood, are typically first-generation antipsychotic medicines, such as haloperidol. These may also cause gynaecomastia (benign enlargement of the one or both male breasts) and galactorrhoea (milk production from the breast unrelated to pregnancy or breast feeding) but will not cause headaches or visual field defects as there is no mass effect. Second-generation antipsychotics and antidepressants, such as risperidone and paliperidone, may cause a modest but clinically insignificant elevation in prolactin[11]. Acute illness, malnutrition, eating disorders, obesity, autoimmune disorders and alcoholism can all result in functional hypogonadism[8].
Opioids can cause hypogonadism through three main mechanisms.
- They bind to receptors in the hypothalamus resulting in reduced GnRH secretion[11];
- They can also act directly on the testes and pituitary gland;
- They can cause raised prolactin which in turn inhibits FSH and LH release[11].
A dose–effect relationship has been demonstrated with higher doses of opioids resulting in lower serum testosterone[12]. Intrathecal opioids and methadone are the most harmful with buprenorphine, a partial opioid agonist, having more modest effects[11].
Androgenic anabolic steroids are a class of drugs that include testosterone and its derivatives. High-circulating testosterone causes reduced LH and FSH levels via negative feedback. This results in loss of the FSH stimulus on Sertoli cells and of LH on Leydig cells[13]. This leads to a paradoxical situation whereby serum testosterone is raised while intratesticular testosterone is suppressed to an extent that spermatogenesis is impaired and testicular atrophy occurs. Concurrent use of human chorionic gonadotropin (hCG) allows preservation of testicular volume[14]. Following cessation of androgenic anabolic steroids, there is a delay in return to baseline of serum gonadotropins and testosterone with the former returning to baseline levels within 13–24 weeks, while testosterone may remain reduced at 16 weeks[15]. Exogenous corticosteroids can suppress testosterone production by inhibiting hypothalamic GnRH secretion and by directly suppressing gonadal steroid secretion[16]. Pharmacists can caution patients about the adverse impact of androgenic steroids on male fertility.
Defects in sperm transportation
Impaired sperm transportation may result in oligospermia (low levels of sperm in the ejaculate) or azoospermia (no sperm in the ejaculate). Azoospermia can be classified into obstructive or non-obstructive. Obstructive causes account for 15-20% of cases and include infection, transection of the vas following vasectomy and congenital absence of the vas deferens (the tube that carries the sperm from the epididymis to the urethra), which may be associated with cystic fibrosis[4]. Alpha blockers used for the treatment of lower urinary tract symptoms can cause contraction of the seminal vesicles resulting in a lower ejaculate volume[17]. Silodosin, a highly selective alpha 1A-blocker, induces reversible failure of ejaculation[18].
Teratogenicity of certain medications
Patients need to be aware of the uncertainty regarding the potential teratogenicity of certain medicines. Guidance from the Medicines and Healthcare products Regulatory Agency (MHRA) advises to discontinue mycophenolate mofetil prior to conception[19]. However, the data informing this recommendation are limited and patients should be advised to see their specialist team for advice.
Cyclophosphamide and thalidomide, which are immunomodulatory drugs used in rheumatology, should be stopped 12 and 4 weeks, respectively, prior to attempting conception[20]. Azathioprine, colchicine, hydroxychloroquine and tumour necrosis factor inhibitors can all be safely continued[20]. Specialist advice should be sought regarding methotrexate, tacrolimus, sulfasalazine, leflunomide and cyclosporin when used to treat in rheumatology[20].
Clinical features
Most men with infertility will have no symptoms and will first present with difficulty conceiving[21]. Some may present with delayed puberty, undescended testes, low libido, erectile dysfunction, reduced need to shave, loss of muscle bulk or anaemia[22].
Reduced libido, reduction in spontaneous early morning erections, fatigue and reduction in facial hair may be suggestive of hypogonadism[22]. Headaches and visual field defects may occur if there is a pituitary lesion. Loss of smell is suggestive of Kallmann syndrome[9].
Screening for symptoms of infection such as blood or pus in the sperm are essential. Cloudy post coital micturition is suggestive of retrograde ejaculation. This may occur in diabetes mellitus secondary to autonomic dysfunction[23]. Other symptoms that may be suggestive of this include postprandial fullness, vomiting, chronic diarrhoea or constipation[23].
As noted above, systemic chemotherapy and pelvic irradiation can irreversibly damage testicular germ cells and this should be considered during history taking, although sperm cryopreservation is usually offered prior to gonadotoxic oncologic treatments[5]. Previous testicular trauma or surgery, including orchidopexy or orchidectomy, should be ruled out. Hernia repair and bladder surgery can result in injury to the spermatic cord and should be considered.
Diagnosis and assessment
There are no validated questionnaires or assessment tools to identify at risk patients for male infertility — examination and investigation are essential. Prior to this, patients may present to pharmacies to purchase antioxidants, which may be their first contact with healthcare professionals. Pharmacists can discuss general lifestyle information and review any medicines that may interfere with fertility. They should direct patients to their GP for examination and initial investigations. Depending on the findings, referral to urology, andrology or fertility services may be required.
For those presenting with subfertility, history should include enquiring about pregnancies from current or previous relationships, duration of subfertility and frequency of sexual intercourse. Just over 80% of couples will conceive within one year of trying with a further 10% within the second year[1]. There is only a marginal increase in pregnancy rate thereafter, with just 3% of couples conceiving between the second and third year[1]. A full drug history should also be taken. Medication including selective serotonin reuptake inhibitors can interfere with fertility by affecting libido[24].
Obesity can also contribute to subfertility[4,25]. Clinical examination should assess for height, weight and waist-to-hip ratio. Patients with Klinefelter syndrome are typically tall, while those with hypogonadotropic hypogonadism may be of shorter stature if the patient has concomitant growth hormone deficiency[26].
Examination should include assessment for hypogonadism, lesions suggestive of testicular defects and obstructive causes. Findings suggestive of hypogonadism are abnormal secondary sexual characteristics, such as decreased body hair, gynaecomastia and low muscle volume[21]. Low testicular volume as assessed with a Prader orchidometer is also suggestive of hypogonadism[21].
Examination should also assess for testicular defects like undescended testes and testicular lesions, such as varicocoeles (enlarged veins in the scrotum), which can also contribute to subfertility. Obstructive causes should also be looked for; absence of the vas deferens can also be assessed on palpation and, if bilateral, can be expected to cause azoospermia[21].
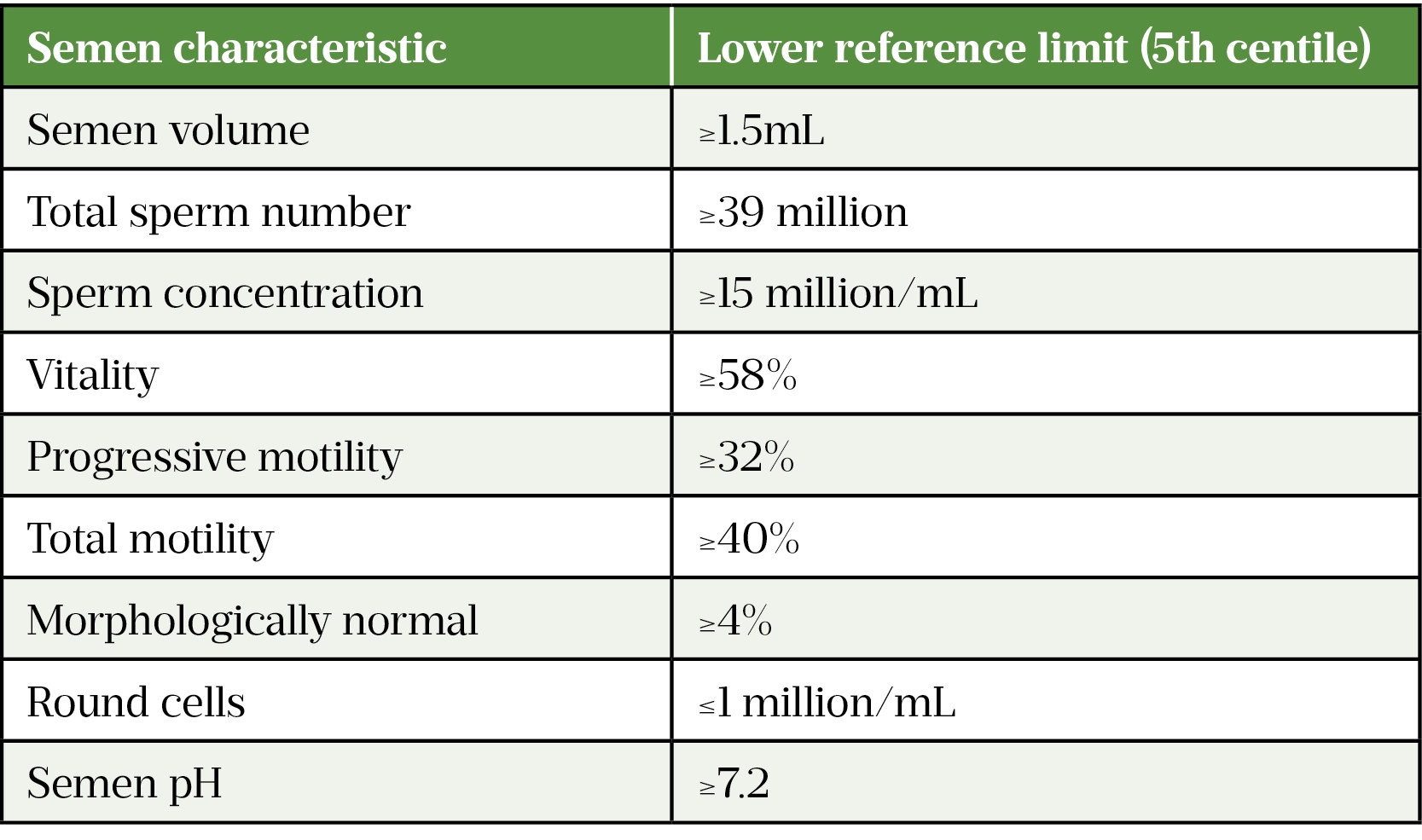
Semen analysis is a crucial investigation. The World Health Organisation diagnostic criteria are derived from more than 4,500 males in 14 countries whose partners had a time-to-pregnancy of under 12 months[27]. An abnormal semen analysis is defined as having one or more semen parameters below the 5th centile (see Table 2). A follow-up hormone profile should be arranged to establish the patient’s levels of FSH, LH, testosterone and prolactin. Testosterone levels exhibit a circadian variation with peak levels in the morning[28]. Levels are reduced in the evening and after meals[29].
Testicular ultrasound is recommended in patients with azoospermia or oligospermia[30]. A pituitary MRI may be considered if prolactin is raised, the hormone profile is suggesting of hypogonadotropic hypogonadism, or there are symptoms suggestive of a pituitary lesion. Karyotype abnormalities are seen in 12–15% in men with azoospermia and 5% severe oligospermia and so this should also be checked for any patients presenting with these conditions[21].
Box 1: Terminology used in World Health Organization (WHO) diagnostic semen analysis
- Normozoospermia: All semen parameters normal;
- Oligozoospermia: Reduced sperm numbers;
- Asthenozoospermia: Sperm motility decreased;
- Oligoasthenozoospermia: Sperm numbers and motility reduced;
- Teratozoospermia: Increased abnormal forms of sperm;
- Aspermia: No ejaculate;
- Azoospermia: Sperm absent;
- Leucocytospermia: Increased white cells in semen.
Infertility can have a significant impact on mental health. The impact on women diagnosed with infertility is well-established, whereas research on men diagnosed with infertility is lacking[31]. However, general research suggests that men are less likely to access support when faced with an emotionally challenging situation[31]. Sensitive communication exploring patient concerns while respecting cultural perceptions surrounding fertility is important. The focus in fertility is often on the female experience and, in some cases, male infertility is not openly discussed[31]. Making patients aware of its high prevalence can be liberating for men who may feel stigmatised by their diagnosis. Pharmacists are optimally placed to signpost patients to available support and counselling services.
Management
Management of male infertility should be individualised, taking into account the underlying aetiology, the partner’s age, causes of female factor infertility, cultural and religious beliefs and eligibility for NHS-funded assisted conception treatment. Management may be conservative, medical or surgical.
Conservative management
When attempting to optimise fertility, men should avoid smoking, any medicines that may negatively impact on semen quality (e.g. anabolic steroids), high doses of corticosteroids, opioids and excessive alcohol. Lifestyle modification, particularly with attention to maintaining a healthy body weight, is essential. Strategies to improve fertility are summarised in Box 2. When trying to conceive, couples are encouraged to have regular intercourse every two to three days, increasing to one to two days in the six days surrounding ovulation[32].
Box 2: Strategies to improve fertility
- Regular exercise and healthy dietary pattern to maintain body mass index;
- Smoking cessation;
- Avoidance of anabolic steroids and illicit drugs;
- Limit alcohol consumption;
- Treatment and control of chronic diseases, such as diabetes;
- Ensure regular medication is not teratogenic and does not impact on semen quality parameters.
Antioxidants are available over the counter and have been marketed as improving male fertility by targeting semen oxidative stress. However, study data are conflicting: the MOXI randomised clinical trial did not show a difference in sperm concentration, motility, morphology, DNA fragmentation or live birth following three to six months of antioxidants (vitamin C, vitamin E, selenium, l-carnitine, zinc, folic acid, lycopene) versus placebo[33]. Similarly, in a randomised control study of couples undergoing IVF for male factor infertility, pre-treatment for three months with oral antioxidants (vitamin C, vitamin E and zinc) versus placebo did not result in superior semen analysis parameters, higher clinical pregnancy or live birth rates[34]. Results from a 2019 Cochrane meta-analysis suggested that antioxidant use may result in an increased live birth rate but cautioned about the quality of included studies[35]. Pharmacists can discuss the evidence regarding multivitamins and nutritional supplements and can deter patients from costlier non-evidence-based preparations.
Medical
There are several causes of male infertility that can be treated medically.
Medical therapy for erectile dysfunction
Penile erection relies on smooth muscle relaxation. This is mediated through the action of cyclic guanosine monophosphate (cGMP), which ultimately results in lower intracellular calcium and subsequent relaxation of arterial and trabecular smooth muscle, leading to arterial dilatation, venous constriction and the subsequent rigidity of penile erection[36].
Medical treatments are aimed at inducing smooth muscle relaxation and include two main classes of medicine: phosphodiesterase type-5 inhibitors (PDE5is) and synthetic forms of prostaglandin E1 (PGE-1). PDE5i induce smooth muscle relaxation by inhibiting PDE5, which would ordinarily degrade cGMP. By contrast, alprostadil works through activation of the cAMP pathway. Contrary to PDE5i, an erectogenic stimulus is not required for Alprostadil to be effective[37].
Unlike alprostadil, PDE5is are available over the counter and are, overall, very safe with few contra-indications, namely concurrent use of nitrates and inadequate cardiac reserve[39]. The effectiveness is in the range of 56–84%[37]. Typical doses are sildenafil 50–100 mg, tadalafil 5–20 mg, vardenafil 10–20 mg, and avanafil 50–200 mg[38–42]. Reported side effects include headache, facial flushing, muscle aches, nasal congestion, blue tinge in vision, dizziness, dyspepsia and priapism[38].
Alprostadil is available through secondary care and is administered via the intracavernosal route[43]. Combination therapy with both PDE5Is and alprostadil is effective in patients who do not respond to monotherapy[37].
Medical therapy for hypogonadotropic hypogonadism
HH invariably results in low testosterone, which has implications for both long-term health and fertility. Outside the realm of fertility, hormone replacement therapy in the form of intramuscular or transdermal testosterone is sufficient[8]. This, however, can interfere with fertility via the negative feedback of testosterone on LH and FSH, which are both required for spermatogenesis[8,25]. Patients should see their specialist when they decide they want to conceive in order to stop or switch to an alternative medicine. A surprisingly high number of men prescribed testosterone are unaware of this, so the pharmacist is central to ensuring that all men are aware of the need to temporarily switch from testosterone when starting a family.
Induction of spermatogenesis is performed via specialist clinics. Considering the hypothalamo-pituitary axis (see Figure), this can theoretically be achieved with either exogenous GnRH acting on the pituitary and thereafter the testes, or with medication with FSH/LH-like activity, which act directly on the testes[44]. This mimics the natural process of spermatogenesis[44].
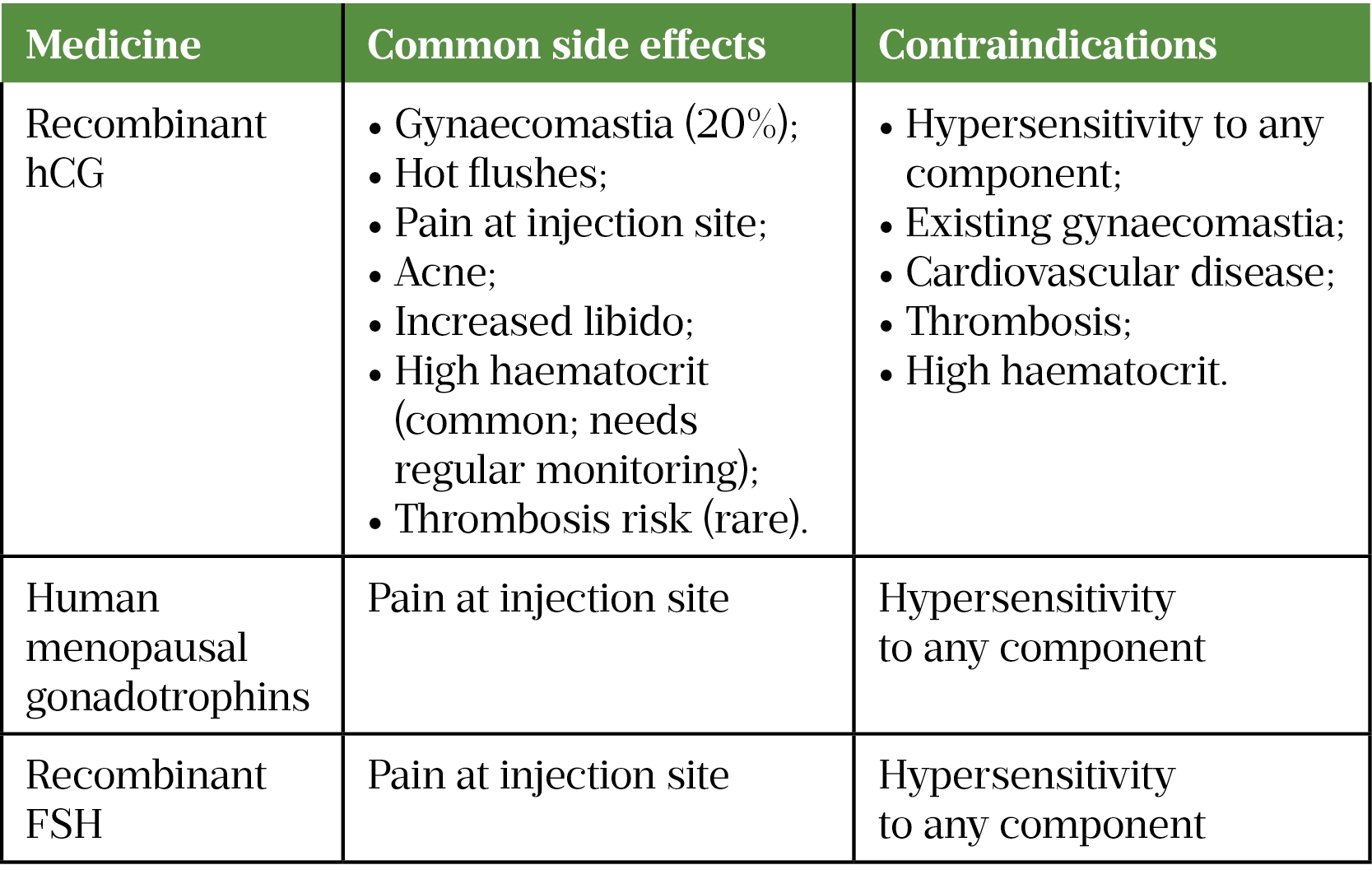
There are a number of commercially available preparations. hCG has an identical alpha subunit as LH and therefore has LH activity binding to receptors on Leydig cells to produce testosterone[25,45]. Recombinant FSH and human menopausal gonadotropin (hMG) are often included in treatment protocols and are classed as gonadotropins[7].
While in theory GnRH can be used for sperm induction, this treatment is costly, difficult to administer and ineffective in those with pituitary disease[44]. The mainstay therapy is therefore with gonadotropins[25]. This is the only licensed fertility therapy for men with HH[25].
Spermatogenesis induction is performed using human chorionic gonadotropin (hCG) with or without rFSH or hMG[7,25]. Dosing regimens vary with doses of 500–2,000IU two to three times a week via subcutaneous injections[25]. Response to treatment is monitored via four to six weekly testosterone levels aiming for a serum testosterone within the normal range[7]. Gynaecomastia and polycythaemia with the inherent risk of thrombosis can occur so estradiol levels and haematocrit should be monitored closely (see Table 3)[7]. Importantly, as testicular volume increases in response to treatment, dose reduction may be required to prevent supraphysiological testosterone levels[7]. Semen analysis is checked at three months of treatment[25]. Positive predictive factors of response to treatment include larger pre-treatment testicular volume[8,46].
Spermatogenesis is successfully achieved in 80% of patients undergoing sperm induction for HH[8]. Sperm recovery is observed at four to six months with normal serum testosterone at six months but with pregnancy usually occurring as late as two to three years[4,8]. With this in mind, some advocate continuing this treatment regime after conception if the couple wish to have another child within two years[4]. Alternatively, semen cryopreservation can be considered if they wish to discontinue treatment. For those achieving only modest response to treatment and those couples who do not conceive in spite of normal semen analysis, assisted conception treatment via IVF/ICSI may be required.
Those with pre-pubertal GnRH deficiency (i.e. occurring before the onset of puberty) are unlikely to respond to hCG monotherapy[4,46,47]. This group is also more likely to have lower testicular volume and higher inhibin levels. rFSH or hMG is usually added to their treatment regime with a starting dose of 75 units two to three times a week, titrating up accordingly. Both rFSH and hMG have FSH activity, but the former is a considerably more expensive formulation with no evidence of greater effectiveness[44,48] with doses going as high as 300 units three times a week[21,46]. Prolonged treatment and with higher doses is often required for those with pre-pubertal GnRH deficiency and in spite of this, they often achieve only modest improvements in semen parameters[7,46].
A novel approach to poor prognosis patients, namely those with congenital HH is with ‘FSH priming’[7]. In this treatment regime, FSH is given for two to four months prior to the introduction of combined FSH and hCG. This protocol aims to mimic a more physiological approach. Initial FSH monotherapy aims to maximise the growth of Sertoli cell and seminiferous tubules prior to the introduction of combined hCG and FSH for spermatogenesis[7]. Large scale studies are required to validate this approach.
Medical therapy for oligospermia and non-obstructive azoospermia
Medical therapy outside the realm of hypogonadotropic hypogonadism remains controversial. Treatment with selective estrogen receptor modulators (SERMs), aromatase inhibitors and hCG has been explored in patients with oligospermia and in those with non-obstructive azoospermia prior to testicular sperm extraction. If the patient has raised levels of gonadotropins prior to treatment, then testicular failure is likely. In this situation, medical therapy is unlikely to be effective[4]. The treatment rationale is to increase intra-testicular testosterone and stimulate spermatogenesis to improve the likelihood of successful surgical sperm retrieval for subsequent use in IVF/ICSI.
SERMs are competitive partial agonists of the estrogen receptors. They have different actions on estrogen receptors in different tissues thereby having a pro-estrogenic effect in some and anti-estrogenic in others[49]. Clomiphene and tamoxifen are conventionally used for ovulation induction and breast cancer respectively[50,51]. In male fertility, they have been used in hypoandrogenic men to block the negative inhibition of estrogen on the pituitary gland thereby resulting in higher FSH and LH levels[52]. This should in theory stimulate Leydig cells to produce higher intra-testicular testosterone levels and trigger spermatogenesis in Sertoli cells[52]. Clomiphene is used at doses of 25–50mg once daily with Tamoxifen used at 20–30mg once a day[52]. Their use in male infertility is off label. Clomiphene use is not without risk. Patients should be counselled about the small, but significant, risk of thrombosis[53].
Oligoteratozoospermia is often encountered in andrology clinics (see Table 3). Two recent meta-analyses looked at the effectiveness of clomiphene on spontaneous conception in couples with oligo- and/or astheno- and /or teratozoospermia. Both studies showed higher pregnancy rates and improved semen parameters in the intervention group[39,40]. However, both studies cautioned about the quality of included trials.
Aromatase inhibitors (AIs) act on the cytochrome P450 enzyme aromatase to prevent the conversion of androgens to estradiol and thereby results in a higher testosterone to estradiol ratio. Given the importance of estradiol for gaining and maintaining bone mass, there are concerns that long-term AI use may have a detrimental effect on bone mineralisation[54]. This is an important consideration given the off-license use of this medication in the treatment of male factor infertility. Other side effects — although usually self-resolving and not clinically significant — are nausea, stomach discomfort, headache, loss of libido, hair loss, weight gain, increase in live enzymes, constipation, diarrhea, bad sleep, urinary frequency, nose bleeds, breast tenderness, irritability/depression/nervousness, joint and tendon pains and swollen limbs[54]. Most studies used letrozole 2.5mg or anastrozole 1mg for between three months to one year.
Regarding AIs, good quality published data are limited with only two randomised controlled trials looking at the role of AIs in male subfertility[55,56]. Evidence suggests that in oligospermic men with low testosterone or low testosterone to estradiol ratios (less than 10:1) but normal FSH and LH, there is a decrease in serum estradiol with an increase in testosterone, total motile count and sperm concentration but not sperm motility. Importantly, there appears to be a correlation between changes in testosterone to estradiol ratio and improvement in total motile count[57]. A 2020 meta-analysis showed an improvement in hormone and semen analysis parameters; however, the researchers cautioned about the need for randomised controlled trials[58]. This was also confirmed in a 2022 review of published studies[59]. The American Association of Urologists suggest that SERMs, AIs, hCG or a combination of the above may be used for infertile men with low serum testosterone[60].
The long-term safety of AIs and SERMs in men is not fully understood. Pharmacists should be vigilant for reports of side effects and use the yellow card system where appropriate.
Where medical and surgical treatment fail, assisted conception using donor sperm can be considered[60]. This is beyond the scope of this review.
Surgical
Surgical sperm retrieval is the mainstay of treatment for obstructive azoospermia with success rates of 100%[61]. It can also be used for non-obstructive azoospermia with significantly lower success rates depending on the underlying diagnosis. Urological input should be sought.
For those in whom the above treatments are unsuccessful or contraindicated, assisted conception with in vitro fertilisation with or without intra-cytoplasmic sperm injection may be employed. This is beyond the scope of this article.
For those undergoing surgical sperm retrieval (SSR) for non-obstructive azoospermia, there is a correlation between normal serum testosterone levels and successful sperm retrieval[62]. Studies have explored the role of hormone therapy prior to surgical sperm retrieval. Groups that may benefit from hormone pre-treatment prior to SSR are those with: low basal testosterone but normal FSH and LH; and those with previous histological diagnosis of maturation arrest of hypospermatogenesis[62]. Treatment regimens vary among studies and include clomiphene citrate 25–75mg/day, hCG 5,000 units three times a week +/- FSH 150 units twice a week, FSH 75IU three times a week and hMG[63–65]. Doses are titrated aiming for normal serum testosterone levels. Use of this medication is off license and the American Association of Urologists suggests that patients should be made aware that the data supporting pharmacological treatment is limited[60]. The European Association of Urology does not advocate the use of hormone therapy in men with NOA[30].
Three small studies support the use of AI for azoospermia demonstrating sperm in the ejaculate post AI treatment[66–68]. This was not replicated in follow-up studies[57,69].
Conclusion
Male infertility is relatively common and should be approached in a sensitive manner. Medication can contribute to male factor subfertility through different mechanisms and pharmacists should be vigilant of this. Whilst antioxidants are safe, the evidence base supporting their use as a means of enhancing fertility is limited. The only licensed male medical treatment is for hypogonadotropic hypogonadism. Use of SERMs, AIs and hCG for oligospermia or prior to surgical sperm retrieval for non-obstructive azoospermia is off-license and with limited evidence to support its use.
Useful resources
Counselling services
- All fertility clinics licensed by the Human Fertilisation and Embryology Authority (HFEA) offer counselling. This may be free or at a cost;
- GPs can arrange for referral to counselling;
- Private counselling can be arranged through The British Infertility Counselling Association (BICA).
Support services
- Men’s Health Forum — an online community where men can chat anonymously;
- HIMFertility Campaign — a website dedicated to encouraging men to talk about fertility problems and signpost them and their partners to much-needed support.
SPONSORED FEATURE: Phosphodiesterase 5 receptor (PDE5) inhibitors and the treatment of erectile dysfunction
Article commissioned and fully funded by Sanofi Consumer Healthcare
- 1Infertility. National Institute for Health and Care Excellence. 2018.https://cks.nice.org.uk/topics/infertility/ (accessed Jul 2022).
- 2Jung JH, Seo JT. Empirical medical therapy in idiopathic male infertility: Promise or panacea? Clin Exp Reprod Med. 2014;41:108. doi:10.5653/cerm.2014.41.3.108
- 3O’Donnell L, Stanton P, de K, et al. endotext. Published Online First: 11 January 2017.http://www.ncbi.nlm.nih.gov/books/NBK279031/
- 4Anawalt BD. Approach to Male Infertility and Induction of Spermatogenesis. The Journal of Clinical Endocrinology & Metabolism. 2013;98:3532–42. doi:10.1210/jc.2012-2400
- 5Brannigan RE, Fantus RJ, Halpern JA. Fertility preservation in men: a contemporary overview and a look toward emerging technologies. Fertility and Sterility. 2021;115:1126–39. doi:10.1016/j.fertnstert.2021.03.026
- 6Jackson G. Erectile dysfunction and cardiovascular disease. Arab Journal of Urology. 2013;11:212–6. doi:10.1016/j.aju.2013.03.003
- 7Swee DS, Quinton R. Managing congenital hypogonadotrophic hypogonadism: a contemporary approach directed at optimizing fertility and long-term outcomes in males. Therapeutic Advances in Endocrinology. 2019;10:204201881982688. doi:10.1177/2042018819826889
- 8Silveira LFG, Latronico AC. Approach to the Patient With Hypogonadotropic Hypogonadism. The Journal of Clinical Endocrinology & Metabolism. 2013;98:1781–8. doi:10.1210/jc.2012-3550
- 9Forni PE, Wray S. GnRH, anosmia and hypogonadotropic hypogonadism – Where are we? Frontiers in Neuroendocrinology. 2015;36:165–77. doi:10.1016/j.yfrne.2014.09.004
- 10Melmed S, Casanueva FF, Hoffman AR, et al. Diagnosis and Treatment of Hyperprolactinemia: An Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism. 2011;96:273–88. doi:10.1210/jc.2010-1692
- 11Reddy RG, Aung T, Karavitaki N, et al. Opioid induced hypogonadism. BMJ. 2010;341:c4462–c4462. doi:10.1136/bmj.c4462
- 12Eshraghi Y, Hanks N, Rooney S, et al. Establishing a Dose-Response Relationship Between Opioid Use and Hypogonadism: A Retrospective Case-Control Study. TOJ. 2021;21:249–53. doi:10.31486/toj.20.0103
- 13Tatem AJ, Beilan J, Kovac JR, et al. Management of Anabolic Steroid-Induced Infertility: Novel Strategies for Fertility Maintenance and Recovery. World J Mens Health. 2020;38:141. doi:10.5534/wjmh.190002
- 14Anawalt BD. Diagnosis and Management of Anabolic Androgenic Steroid Use. The Journal of Clinical Endocrinology & Metabolism. 2019;104:2490–500. doi:10.1210/jc.2018-01882
- 15Christou MA, Christou PA, Markozannes G, et al. Effects of Anabolic Androgenic Steroids on the Reproductive System of Athletes and Recreational Users: A Systematic Review and Meta-Analysis. Sports Med. 2017;47:1869–83. doi:10.1007/s40279-017-0709-z
- 16Mohammed A, Mansour A, Ahmed J. Effect of exogenous glucocorticoids on male hypogonadism. biom rep. 2020. doi:10.3892/br.2020.1319
- 17Revenig L, Leung A, Hsiao W. Ejaculatory physiology and pathophysiology: assessment and treatment in male infertility. Transl Androl Urol 2014;3:41–9. doi:10.3978/j.issn.2223-4683.2014.02.02
- 18Kobayashi K, Masumori N, Hisasue S, et al. Inhibition of Seminal Emission Is the Main Cause of Anejaculation Induced by a New Highly Selective α1A-Blocker in Normal Volunteers. The Journal of Sexual Medicine. 2008;5:2185–90. doi:10.1111/j.1743-6109.2008.00779.x
- 19Mycophenolate mofetil, mycophenolic acid: updated contraception advice for male patients. Medicines and Healthcare products Regulatory Agency. 2018.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/679673/DSU-Feb-PDF.pdf (accessed Jul 2022).
- 20Sammaritano LR, Bermas BL, Chakravarty EE, et al. 2020 American College of Rheumatology Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases. Arthritis Care Res. 2020;72:461–88. doi:10.1002/acr.24130
- 21Agarwal A, Baskaran S, Parekh N, et al. Male infertility. The Lancet. 2021;397:319–33. doi:10.1016/s0140-6736(20)32667-2
- 22Kumar P, Kumar N, Thakur D, et al. Male hypogonadism: Symptoms and treatment. J Adv Pharm Tech Res. 2010;1:297. doi:10.4103/0110-5558.72420
- 23Vinik AI, Maser RE, Mitchell BD, et al. Diabetic Autonomic Neuropathy. Diabetes Care. 2003;26:1553–79. doi:10.2337/diacare.26.5.1553
- 24Jing E, Straw-Wilson K. Sexual dysfunction in selective serotonin reuptake inhibitors (SSRIs) and potential solutions: A narrative literature review. Mental Health Clinician. 2016;6:191–6. doi:10.9740/mhc.2016.07.191
- 25Jayasena CN, Anderson RA, Llahana S, et al. Society for Endocrinology guidelines for testosterone replacement therapy in male hypogonadism. Clinical Endocrinology. 2021;96:200–19. doi:10.1111/cen.14633
- 26Groth KA, Skakkebæk A, Høst C, et al. Klinefelter Syndrome—A Clinical Update. The Journal of Clinical Endocrinology & Metabolism. 2013;98:20–30. doi:10.1210/jc.2012-2382
- 27Boitrelle F, Shah R, Saleh R, et al. The Sixth Edition of the WHO Manual for Human Semen Analysis: A Critical Review and SWOT Analysis. Life. 2021;11:1368. doi:10.3390/life11121368
- 28Crawford ED, Poage W, Nyhuis A, et al. Measurement of testosterone: how important is a morning blood draw? Current Medical Research and Opinion. 2015;31:1911–4. doi:10.1185/03007995.2015.1082994
- 29Gagliano-Jucá T, Li Z, Pencina KM, et al. Oral glucose load and mixed meal feeding lowers testosterone levels in healthy eugonadal men. Endocrine. 2018;63:149–56. doi:10.1007/s12020-018-1741-y
- 30Sexual and Reproductive Health. European Association of Urology. 2022.https://uroweb.org/guidelines/sexual-and-reproductive-health#note_74 (accessed Jul 2022).
- 31Wischmann T, Thorn P. (Male) infertility: what does it mean to men? New evidence from quantitative and qualitative studies. Reproductive BioMedicine Online. 2013;27:236–43. doi:10.1016/j.rbmo.2013.06.002
- 32Briscoe KK. What is the optimal frequency of intercourse for a healthy couple trying to conceive? Evidence-Based Practice. 2014;17:E8. doi:10.1097/01.ebp.0000540847.31842.68
- 33Steiner AZ, Hansen KR, Barnhart KT, et al. The effect of antioxidants on male factor infertility: the Males, Antioxidants, and Infertility (MOXI) randomized clinical trial. Fertility and Sterility. 2020;113:552-560.e3. doi:10.1016/j.fertnstert.2019.11.008
- 34Joseph T, Mascarenhas M, Karuppusami R, et al. Antioxidant pretreatment for male partner before ART for male factor subfertility: a randomized controlled trial. Human Reproduction Open. 2020;2020. doi:10.1093/hropen/hoaa050
- 35Showell MG, Mackenzie-Proctor R, Brown J, et al. Antioxidants for male subfertility. Cochrane Database of Systematic Reviews. 2014. doi:10.1002/14651858.cd007411.pub3
- 36Corbin JD. Mechanisms of action of PDE5 inhibition in erectile dysfunction. Int J Impot Res. 2004;16:S4–7. doi:10.1038/sj.ijir.3901205
- 37Moncada I, Martinez-Salamanca J, Ruiz-Castañe E, et al. Combination therapy for erectile dysfunction involving a PDE5 inhibitor and alprostadil. Int J Impot Res. 2018;30:203–8. doi:10.1038/s41443-018-0046-2
- 38Penzias A, Bendikson K, Butts S, et al. Diagnostic evaluation of sexual dysfunction in the male partner in the setting of infertility: a committee opinion. Fertility and Sterility. 2018;110:833–7. doi:10.1016/j.fertnstert.2018.07.010
- 39Sildenafil 50 mg film-coated tablets. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/7141/smpc#gref (accessed Jul 2022).
- 40Tadalafil 20 mg film-coated tablets. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/8637/smpc#gref (accessed Jul 2022).
- 41Vardenafil 20 mg tablets. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/9658/smpc#gref (accessed Jul 2022).
- 42Spedra 50 mg tablets. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/5331/smpc#gref (accessed Jul 2022).
- 43Caverject 10 micrograms powder for solution for injection. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/2917/smpc#gref (accessed Jul 2022).
- 44Lin J, Mao J, Wang X, et al. Optimal treatment for spermatogenesis in male patients with hypogonadotropic hypogonadism. Medicine. 2019;98:e16616. doi:10.1097/md.0000000000016616
- 45Choi J, Smitz J. Luteinizing hormone and human chorionic gonadotropin: Origins of difference. Molecular and Cellular Endocrinology. 2014;383:203–13. doi:10.1016/j.mce.2013.12.009
- 46Warne DW, Decosterd G, Okada H, et al. A combined analysis of data to identify predictive factors for spermatogenesis in men with hypogonadotropic hypogonadism treated with recombinant human follicle-stimulating hormone and human chorionic gonadotropin. Fertility and Sterility. 2009;92:594–604. doi:10.1016/j.fertnstert.2008.07.1720
- 47Zacharin M, Sabin MA, Nair VV, et al. Addition of recombinant follicle-stimulating hormone to human chorionic gonadotropin treatment in adolescents and young adults with hypogonadotropic hypogonadism promotes normal testicular growth and may promote early spermatogenesis. Fertility and Sterility. 2012;98:836–42. doi:10.1016/j.fertnstert.2012.06.022
- 48Ortac M, Hidir M, et al. Efficacy of follitropin-alpha versus human menopausal gonadotropin for male patients with congenital hypogonadotropic hypogonadism. Turkish Journal of Urology. 2020;46:13–7. doi:10.5152/tud.2019.19177
- 49Cannarella R, Condorelli RA, Mongioì LM, et al. Effects of the selective estrogen receptor modulators for the treatment of male infertility: a systematic review and meta-analysis. Expert Opinion on Pharmacotherapy. 2019;20:1517–25. doi:10.1080/14656566.2019.1615057
- 50Clomid 50mg Tablets. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/961/smpc#gref (accessed Jul 2022).
- 51Tamoxifen 20mg Film-Coated Tablets. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/2248/smpc#gref (accessed Jul 2022).
- 52Chua ME, Escusa KG, Luna S, et al. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: a meta-analysis. Andrology. 2013;1:749–57. doi:10.1111/j.2047-2927.2013.00107.x
- 53Zahid M, Arshad A, Zafar A, et al. Intracranial venous thrombosis in a man taking clomiphene citrate. BMJ Case Reports. 2016;:bcr2016217403. doi:10.1136/bcr-2016-217403
- 54de Ronde W, de Jong FH. Aromatase inhibitors in men: effects and therapeutic options. Reprod Biol Endocrinol. 2011;9:93. doi:10.1186/1477-7827-9-93
- 55Cavallini G, Biagiotti G, Bolzon E. Multivariate analysis to predict letrozole efficacy in improving sperm count of non-obstructive azoospermic and cryptozoospermic patients: a pilot study. Asian J Androl. 2013;15:806–11. doi:10.1038/aja.2013.99
- 56CLARK RV, SHERINS RJ. Treatment of Men with Idiopathic Oligozoospermic Infertility Using the Aromatase Inhibitor, Testolactone Results of a Double-Blinded, Randomized, Placebo-Controlled Trial with Crossover. Journal of Andrology. 1989;10:240–7. doi:10.1002/j.1939-4640.1989.tb00094.x
- 57Shoshany O, Abhyankar N, Mufarreh N, et al. Outcomes of anastrozole in oligozoospermic hypoandrogenic subfertile men. Fertility and Sterility. 2017;107:589–94. doi:10.1016/j.fertnstert.2016.11.021
- 58Busetto G, Del Giudice F, De Berardinis E, et al. A systematic review and meta-analysis of clinical trials implementing aromatase inhibitors to treat male infertility. Asian J Androl. 2020;22:360. doi:10.4103/aja.aja_101_19
- 59Yang C, Li P, Li Z. Clinical application of aromatase inhibitors to treat male infertility. Human Reproduction Update. 2021;28:30–50. doi:10.1093/humupd/dmab036
- 60Schlegel PN, Sigman M, Collura B, et al. Diagnosis and Treatment of Infertility in Men: AUA/ASRM Guideline PART II. Journal of Urology. 2021;205:44–51. doi:10.1097/ju.0000000000001520
- 61JINNO M, OZAKI T, NAKAMURA Y, et al. Predicting sperm retrieval rates in testicular sperm extraction for azoospermia according to endocrine profiles. Reproductive Medicine and Biology. 2005;4:239–45. doi:10.1111/j.1447-0578.2005.00113.x
- 62Caroppo E, Colpi GM. Hormonal Treatment of Men with Nonobstructive Azoospermia: What Does the Evidence Suggest? JCM. 2021;10:387. doi:10.3390/jcm10030387
- 63Hussein A. Clomiphene Administration for Cases of Nonobstructive Azoospermia: A Multicenter Study. Journal of Andrology. 2005;26:787–91. doi:10.2164/jandrol.04180
- 64Kato Y, Shiraishi K, Matsuyama H. Expression of testicular androgen receptor in non-obstructive azoospermia and its change after hormonal therapy. Andrology. 2014;2:734–40. doi:10.1111/j.2047-2927.2014.00240.x
- 65Aydos K, Ünlü C, Demirel LC, et al. The effect of pure FSH administration in non-obstructive azoospermic men on testicular sperm retrieval. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2003;108:54–8. doi:10.1016/s0301-2115(02)00412-8
- 66Kyrou D, Kosmas IP, Popovic-Todorovic B, et al. Ejaculatory sperm production in non-obstructive azoospermic patients with a history of negative testicular biopsy after the administration of an aromatase inhibitor: report of two cases. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2014;173:120–1. doi:10.1016/j.ejogrb.2013.11.006
- 67Cavallini G, Beretta G, Biagiotti G. Preliminary study of letrozole use for improving spermatogenesis in non-obstructive azoospermia patients with normal serum FSH. Asian J Androl. 2011;13:895–7. doi:10.1038/aja.2011.44
- 68Saylam B, Efesoy O, Çayan S. The effect of aromatase inhibitor letrozole on body mass index, serum hormones, and sperm parameters in infertile men. Fertility and Sterility. 2011;95:809–11. doi:10.1016/j.fertnstert.2010.09.021
- 69PAVLOVICH CP, KING P, GOLDSTEIN M, et al. EVIDENCE OF A TREATABLE ENDOCRINOPATHY IN INFERTILE MEN. Journal of Urology. 2001;165:837–41. doi:10.1016/s0022-5347(05)66540-8