Dr P Marazzi / Science Photo Library
After reading this article the reader should be able to:
- Understand when disease-modifying therapies (DMTs) may be suitable for the treatment of multiple sclerosis (MS);
- Know the different treatment options and when each would be indicated;
- Understand how patients on DMTs are monitored and any considerations for switching.
Multiple sclerosis (MS) is a multifocal progressive autoimmune disease of the central nervous system (CNS). Characterised by nerve demyelination, MS is the most common non-traumatic, disabling neurological disease in young adults in England, and usually presents between the 3rd and 4th decades[1]. The estimated prevalence of MS in England is 190 cases per 100,000, with 105,800 individuals affected, and it is more common in females than in males (2.5:1)[1].
The exact cause of MS remains unknown, but evidence suggests that environmental factors, such as viral pathogens, trigger autoimmune reactions against the myelin that covers nerve cells in genetically susceptible individuals[2].
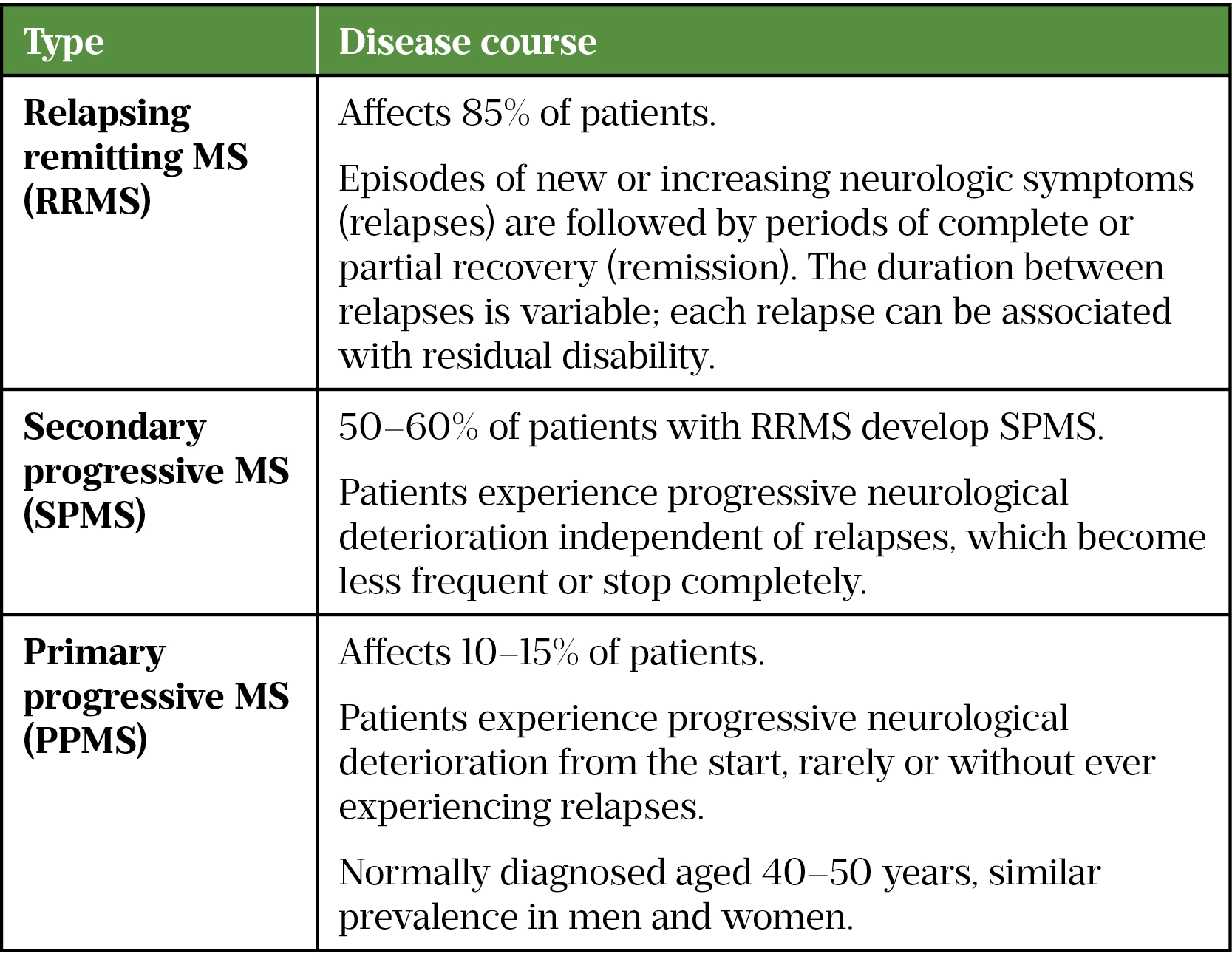
Symptoms vary between patients, depending on the location of nerve damage in the CNS. Common symptoms include optic neuritis, fatigue, spasticity, problems with balance and coordination, difficulty with memory and cognition, bladder and bowel dysfunction, sexual dysfunction and neuropathic pain[2]. These symptoms may have a relapsing presentation followed by periods of partial recovery, but over time progressive disability can occur (see Table 1).
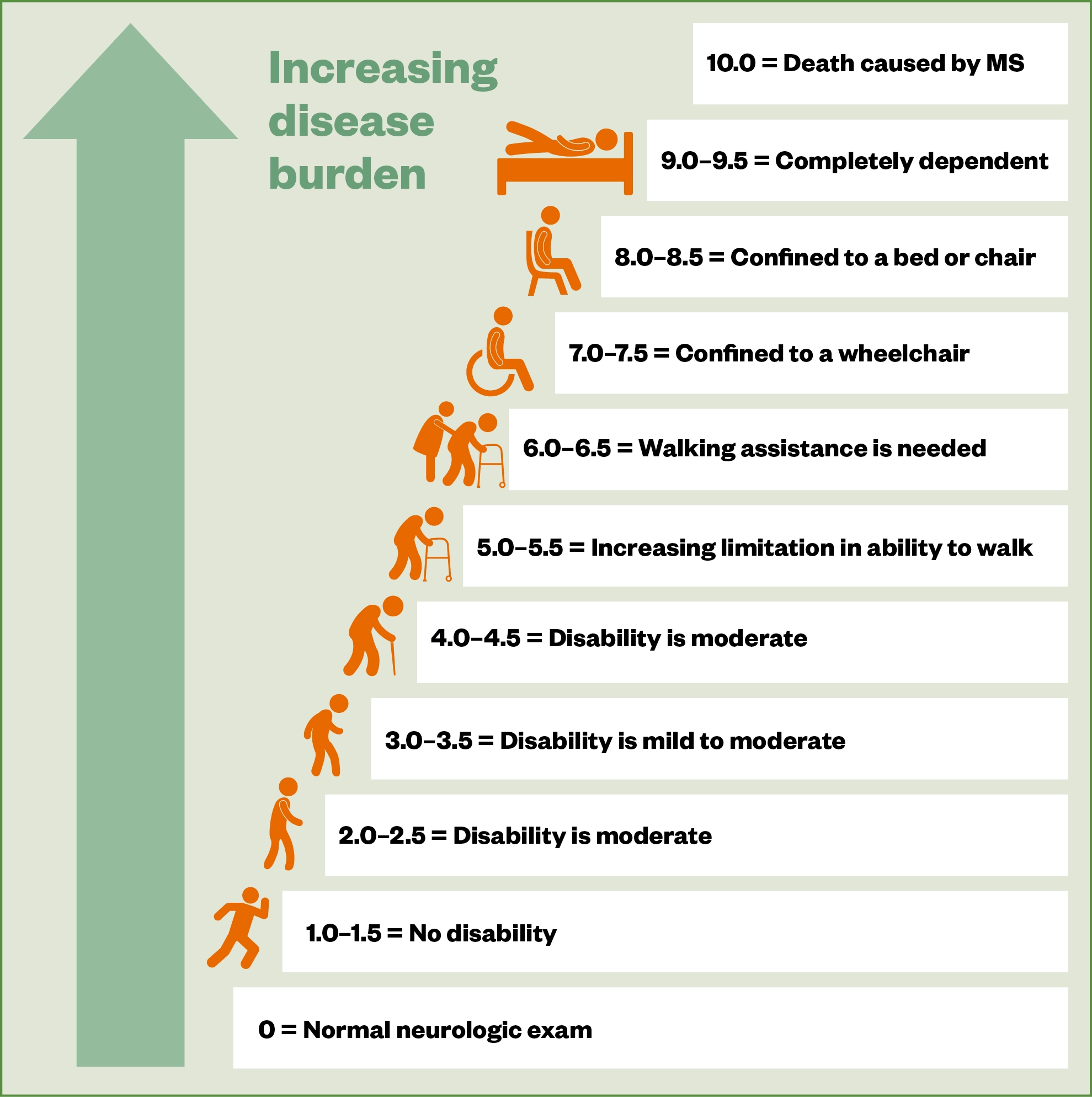
Assessment of disability is made using the ‘Expanded Disability Status Scale’ (EDSS; see Figure 1[2]). Diagnosis is made by a consultant neurologist, based on clinical presentation and MRI activity using established criteria, such as the ‘McDonald criteria’[2–4].
Additional information on symptoms and diagnosis can be found here.
Currently, there is no cure for MS, the mainstay of disease management is disease modifying therapy (DMT), symptom control and supportive therapy, such as physiotherapy.
There has been a significant increase in the number of DMTs available over the past six years, with 12 DMTs currently approved for use in the UK (see Figure 2). Evidence indicates that DMTs should be started early following MS diagnosis to improve long-term outcomes[5,6].
The rapidly evolving landscape of MS treatments is placing increasing pressure on MS services to safely deliver and monitor these medicines. Furthermore, data suggest that a significant number of patients are still not receiving these DMTs, despite access improving, meaning that the workload of healthcare professionals involved in MS care is likely to increase[7].
DMTs are supplied from hospital via homecare or hospital pharmacies; therefore, it is important to establish good links between pharmacists in primary and secondary care. Pharmacists working in primary care should be aware of the supply route for these medicines, as well as how to signpost patients into secondary care services; additionally, they can provide medicines information to patients such as advice on interactions or vaccines.
This article will provide an overview of the available evidence for each DMT and outline each stage of patient care. There are five stages to a patient’s treatment journey: diagnosis and assessment of eligibility, treatment decision, pre-treatment screening, follow up and monitoring and stopping and switching.
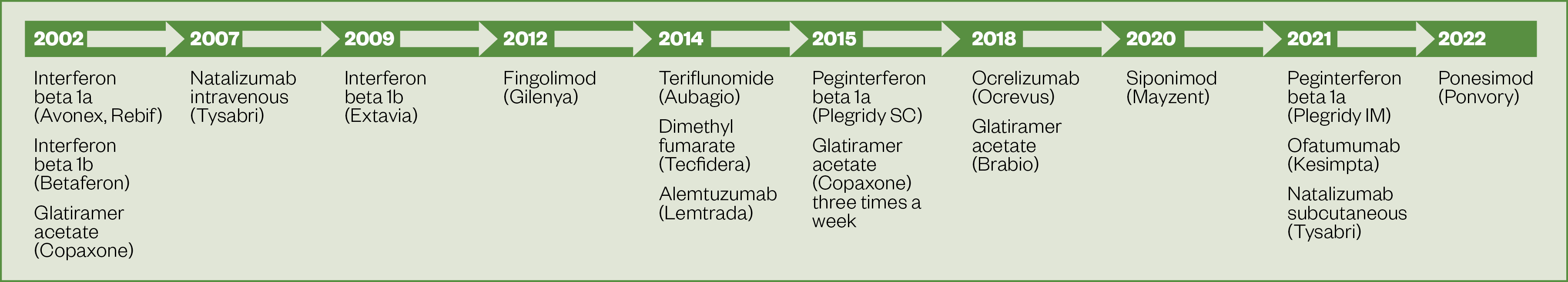
Disease-modifying therapies
In relapsing remitting MS (RRMS), the aim of DMT is to reduce the frequency and severity of relapses; in the long-term, time to disability may be reduced, but most trials have not measured this. The primary endpoint in RRMS trials is reduction in annualised relapse rate (see Table 2[9–26]).
For secondary progressive MS (SPMS) and primary progressive MS (PPMS), the aim of treatment is reduction in risk of progression in disability (see Table 2). The mechanism of action of DMTs is shown in the Box.
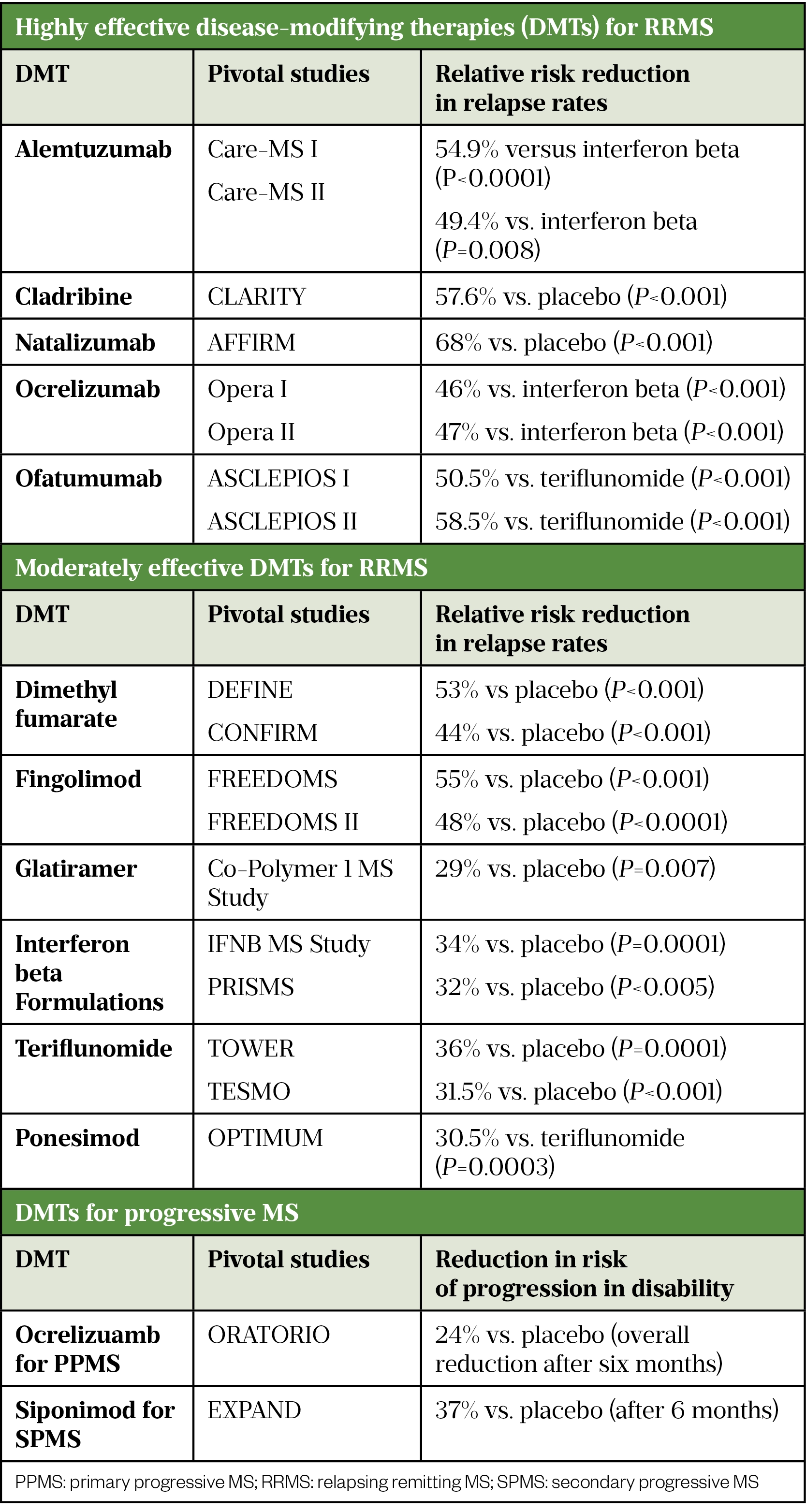
There are two interferon beta-1b treatments licensed for active SPMS (Extavia and Betaferon)[27,28]. Studies have shown inconsistent results for the primary endpoint (time to confirmed progression). Two trials have demonstrated reduction in risk of relapse by 30%[29,30]. One demonstrated a reduction in risk of progression in disability and in time to becoming wheelchair bound; this was not observed in the second trial[30].
A ten-year real world evidence study, which included 4,862 patients, measured the long-term effectiveness of interferon beta and glatiramer acetate. Most patients had RRMS (n=4,217); however, some patients with SPMS were included. This study demonstrated a reduction in progression in disability; the average time to sustained EDSS 6.0 (requiring a walking stick) was 12.5 years verses 8.4 years for untreated individuals[31].
Box: Mechanism of action
The pathophysiology of MS is not currently well understood; however, the adaptive immune system (which includes T and B lymphocytes) is thought to play an important role and therefore is the main target of DMTs, that act by:
- Altering lymphocyte trafficking — natalizumab binds to α4B1 integrin receptor on T cells preventing lymphocytes from crossing the blood brain barrier; fingolimod, siponimod and ponesimod are sphingosine-1-phosphate (S1P) receptor agonists, they bind to S1P receptors on T and B cells, preventing their egress into the blood stream, resulting in retention in the lymph tissue;
- Lymphocyte depletion via cell lysis — alemtuzumab targets CD52 receptors on T and B cells; ocrelizumab and ofatumumab target CD20 receptors on B cells; cladribine is a nucleoside analogue of deoxyadenosine, it is a prodrug targeting T and B cells.
- Disruption of lymphocyte replication — teriflunomide binds to and inhibits the enzyme dihydroorotate dehydrogenase, resulting in reduced proliferation of activated T and B cells[32–36].
The mechanism of action of interferon beta, glatiramer acetate and dimethyl fumarate are not fully understood; they are considered as immunomodulatory (as opposed to immunosuppressant). They act by promoting the regulatory aspects of the immune system, which results in suppression of pro-inflammatory processes[32].
The patient treatment journey
DMTs are prescribed and managed in secondary care by a specialist MS team, including specialist consultants, MS nurses and neurology pharmacists.
Recent studies have demonstrated that there are not enough neurology consultants and MS nurses to provide a long-term sustainable service[37,38]. There is a growing need to use the multidisciplinary team (MDT) to address these issues. Alongside the established roles of pharmacists in managing prescriptions and providing essential medicines information, there are opportunities for pharmacists to support treatment throughout the patient journey, from pre-treatment counselling, prescribing, ongoing monitoring and managing side effects.
Diagnosis and eligibility assessment
In the UK, a patient’s treatment eligibility must be confirmed by a consultant neurologist with a specialist interest in MS and eligibility is dependent on the classification of MS (see Table 2). In England, there are further sub-classifications and specific NHS England commissioning criteria according to disease activity (see Table 3[6,39–50]).
Eligibility for DMTs is normally ratified in an MDT meeting. Under NHS England, this is mandatory for all DMTs apart from lower-risk therapies interferon beta, glatiramer acetate, teriflunomide and dimethyl fumarate[6].
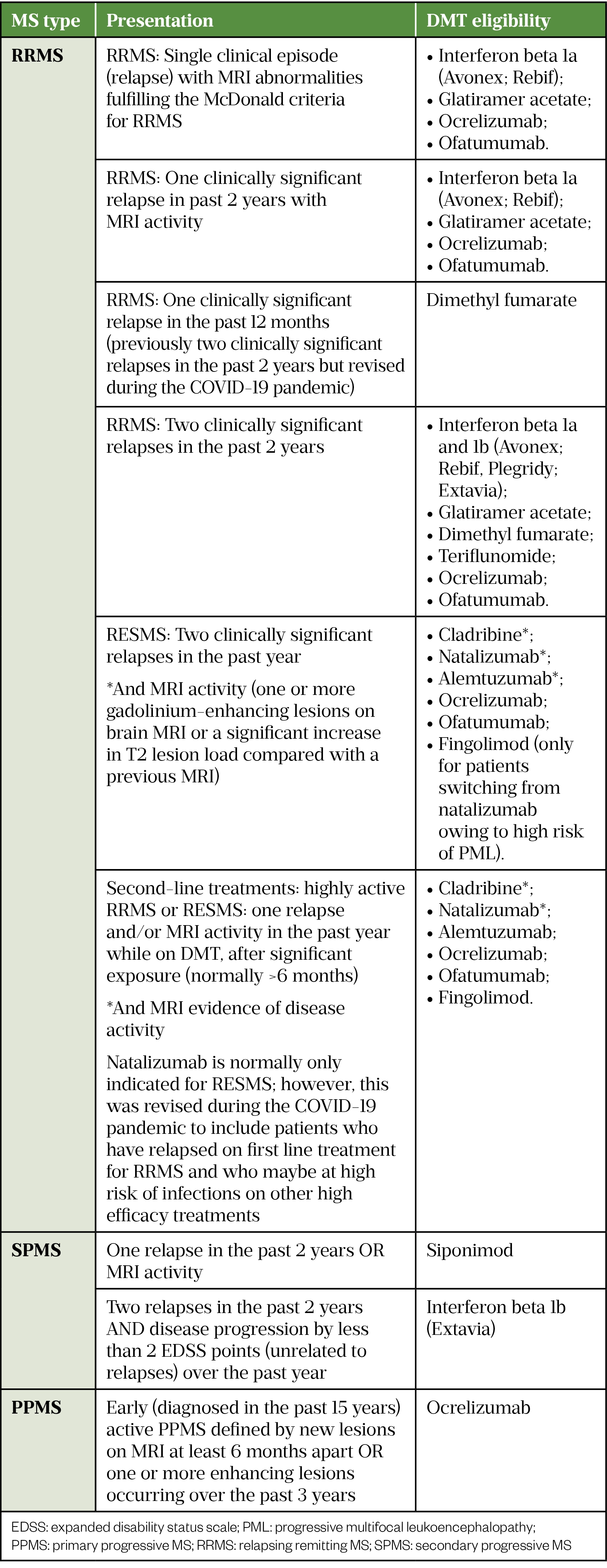
Ponesimod was recently approved by NICE for RRMS, it is yet to be included in the NHS England treatment algorithm for MS DMTs and therefore specific commissioning criteria are not currently described[51].
Treatment decision
Once a decision has been made regarding treatment eligibility, an experienced member of the MS team — normally the MS nurse or pharmacist — will discuss the benefits and risks of all potential options with the patient. DMT choice is individual and based on various factors depending on the patient’s lifestyle and preferences, including:
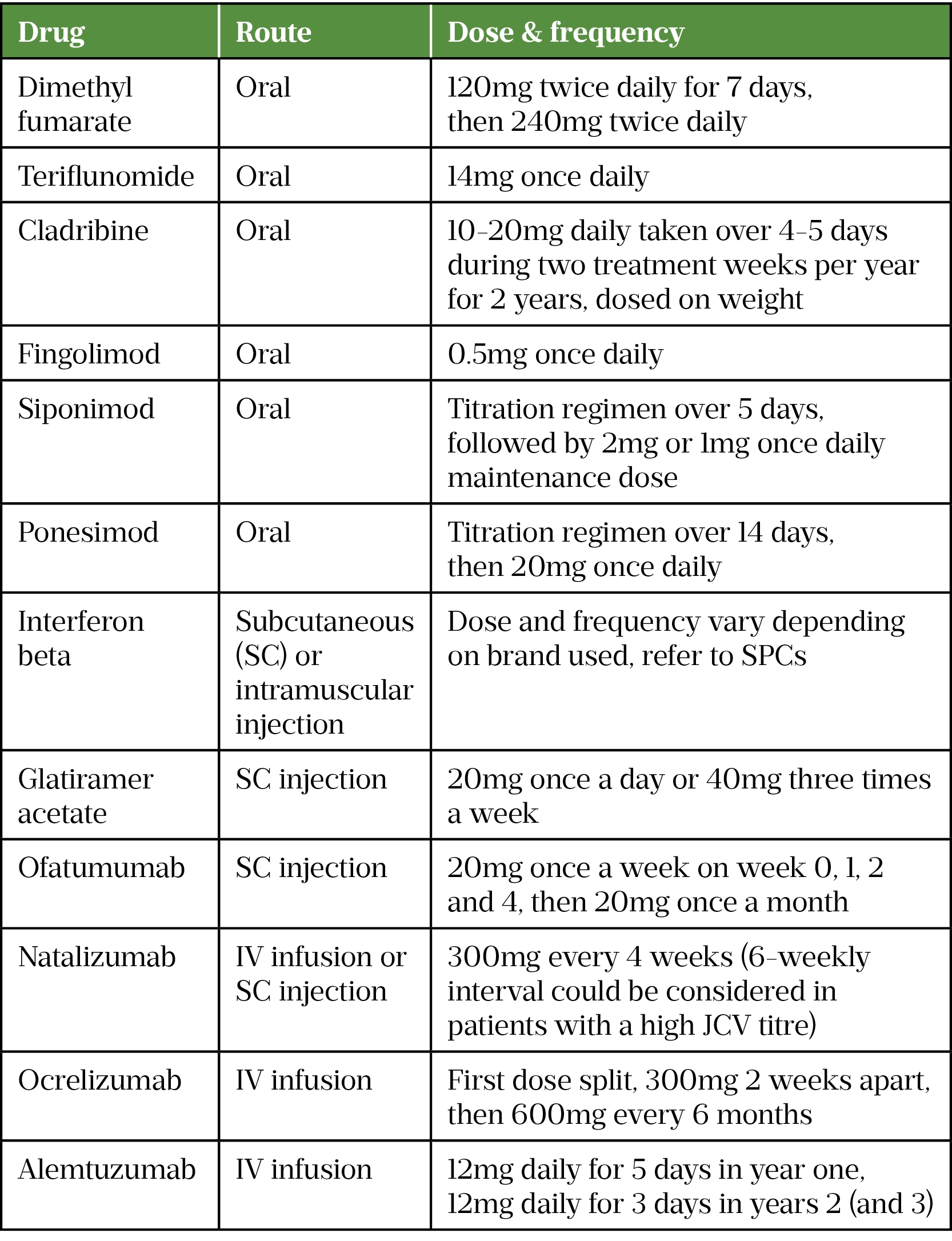
- Route of administration (i.e. oral tablets, self-injection, intravenous infusion) and frequency of dosing;
- Place of administration (natalizumab, ocrelizumab, alemtuzumab and first dose fingolimod all require hospital administration);
- Frequency of blood monitoring;
- Storage requirements (i.e. fridge storage requirements);
- Impact on work and travel;
- Family planning;
- How risk averse the patient is (i.e. balance risk and benefit of each treatment).
Some patients may decide not to start or to delay initiation of DMT for many reasons, including family planning or concerns about potential side effects. It is important to ensure they are aware of the risks of delaying treatment (including relapses and potential impact on long-term disability) and that any barriers they may have are addressed. Patients can be signposted to appropriate resources, such as the MS Trust and MS Society, to support them in making an informed decision[8,52].
Pre-treatment screening
There are general screening tests and history taking that are relevant to all DMTs and completing these early in the treatment journey will help inform decision making.
A full medical and medication history (including vaccine history) should be completed to rule out contraindications and to check for potential interactions. If the patient is already taking a DMT, stopping and switching must be carefully planned.
It is important to further discuss family planning and contraceptive requirements and a pregnancy test should be carried out, where appropriate. The patient should also be made aware of and consent to ongoing monitoring and follow-up requirements.
As DMTs are prescribed in secondary care and may be dosed infrequently, patients should be reminded to include in their medication history to other healthcare professionals they may encounter. Table 5 details the required screening tests for each DMT. Varicella zoster virus antibody screening is not mandated prior to starting all DMTs. However, identification of non-immune patients early in the treatment journey allows timely administration of the live varicella zoster vaccine (2 doses administered 4 weeks apart) and prevents delays in initiation of, or switching between, DMTs in the future. We therefore recommend testing prior to starting all DMTs. If positive it does not require repeat testing for future switching between DMTs.[33–36,53–66].
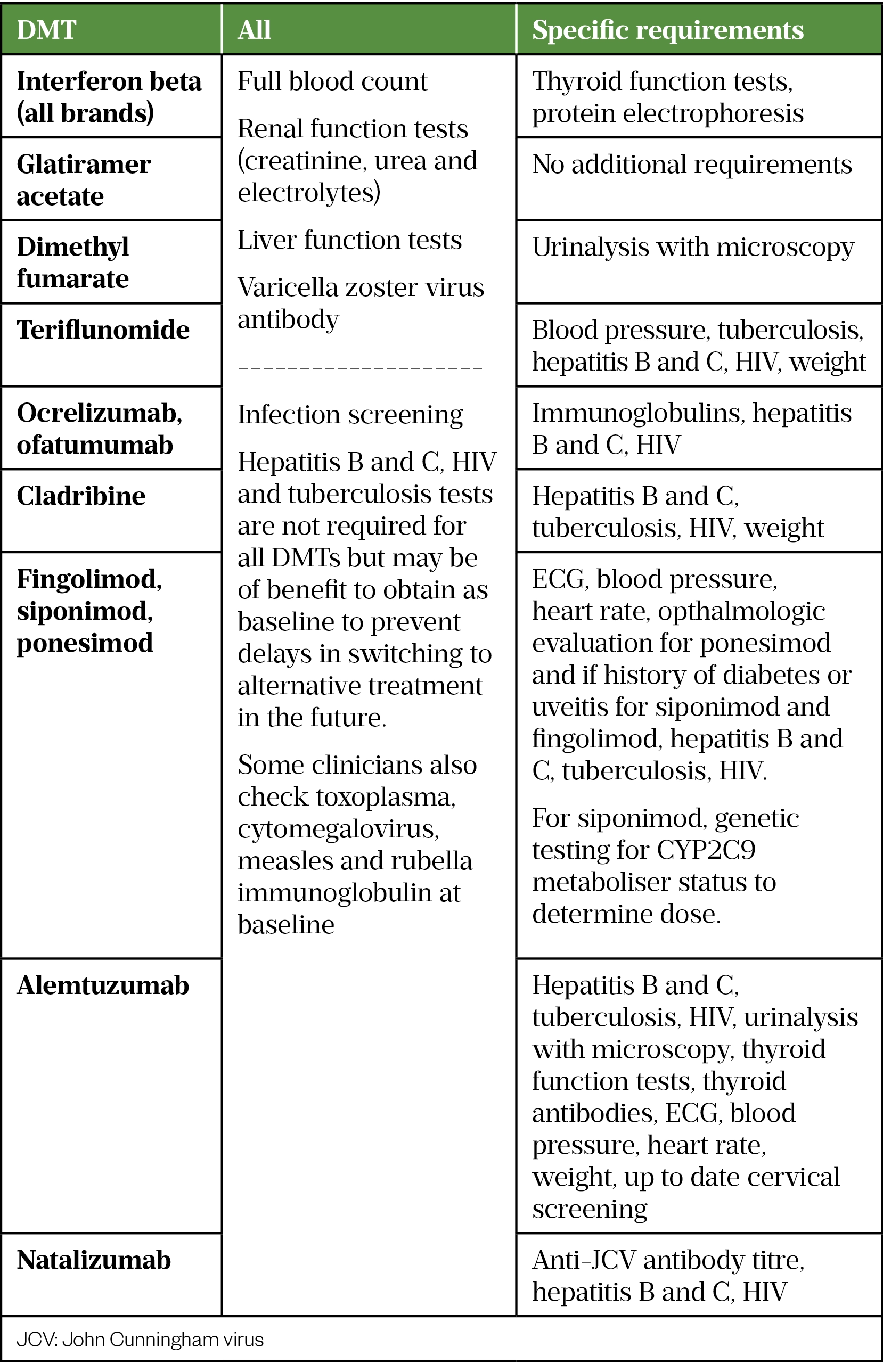
Follow up and monitoring
The MS team are responsible for monitoring safety and response to DMTs. Table 6 describes the minimum monitoring required for each DMT; more frequent blood tests may be required if any abnormalities are identified.
Monitoring recommendations are based on advice from individual summary of product characteristics (SPC), local practice may vary and frequency may currently be reduced owing to advice from the Academy of British Neurologists (ABN) during the COVID-19 pandemic (in order to reduce requirement for hospital attendance). Where monitoring is advised at regular intervals, it is common practice to complete tests every 6-12 months [33–36,53–65].
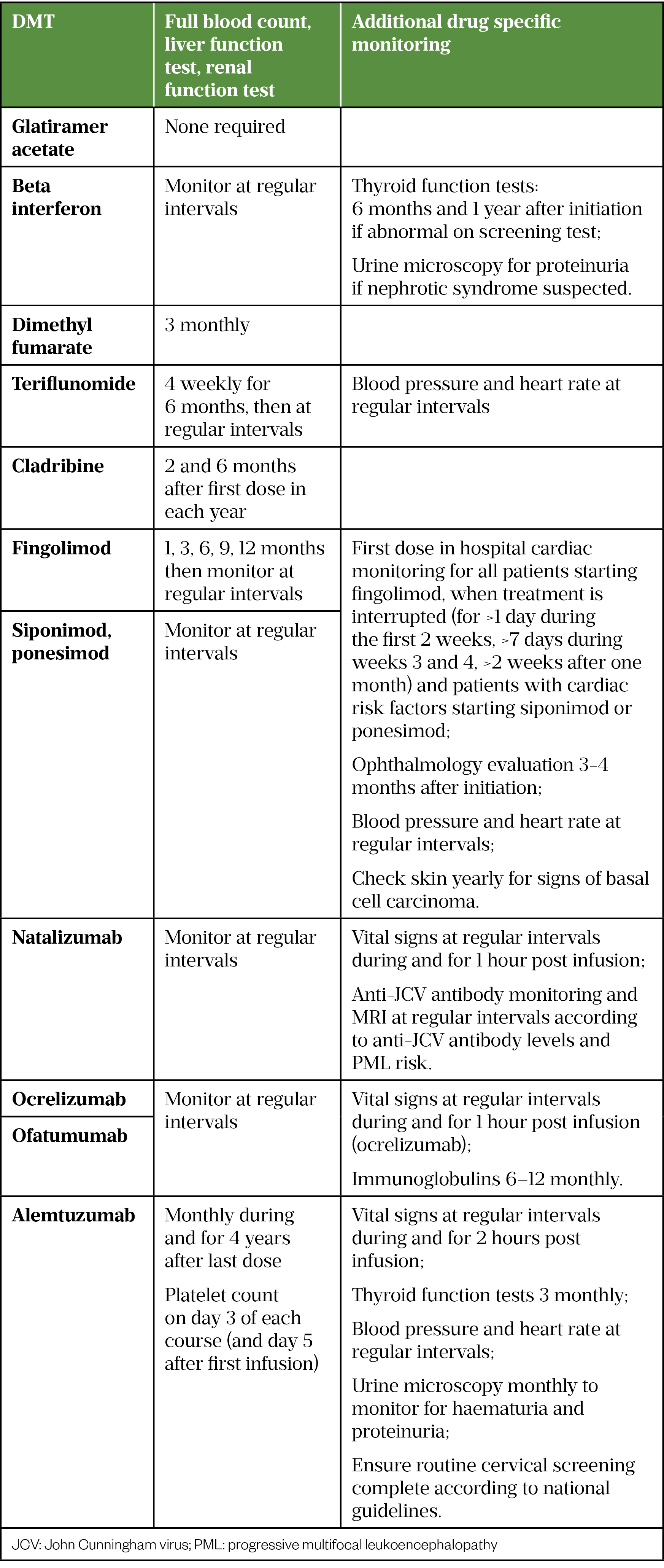
During follow-up consultations, adherence to the medication, side effects, blood monitoring and MS disease progression are discussed to establish tolerance and effectiveness of treatment. Regular MRI scans, assessment of EDSS score and documentation of relapses are an important part of monitoring treatment effectiveness[6]. Ensuring patients understand the long-term goals of treatment is vital in improving adherence[67].
Monitoring and managing side effects
Some of the more common and significant side effects associated with each DMT are summarised in Table 7 (for a full list see individual SPCs). Simple measures may help with some side effects; for example, taking tablets immediately after meals that are high in protein and fat can help reduce gastrointestinal side effects of dimethyl fumarate[61]. With interferons, administering the injection in the evening with regular paracetamol for 24 hours can help alleviate associated flu-like symptoms.
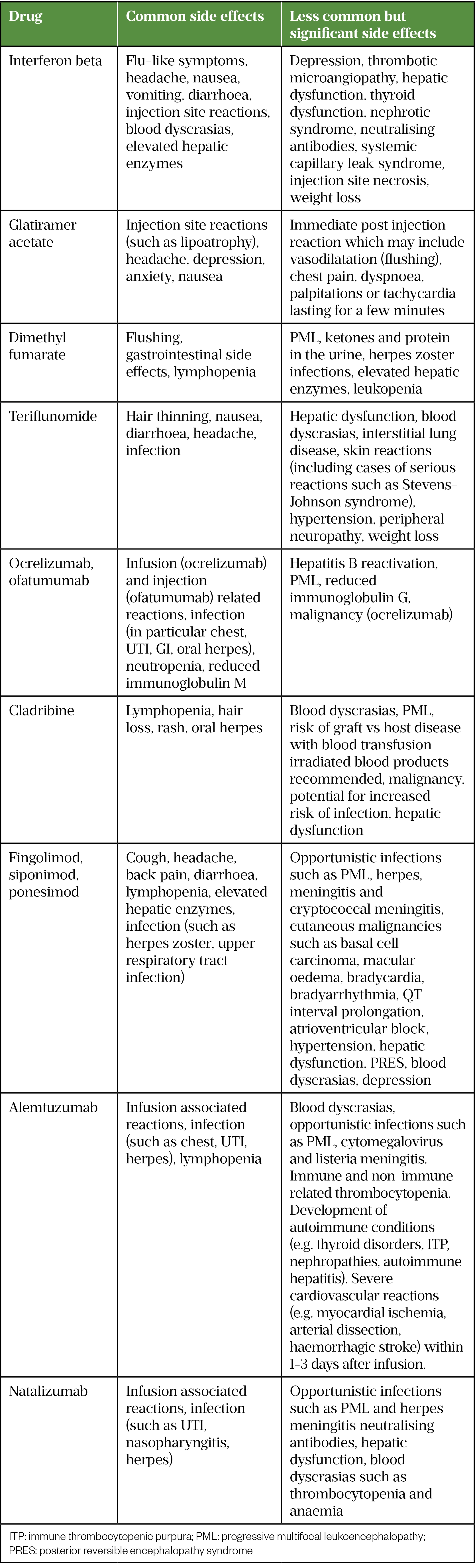
Injection and infusion-related reactions
With subcutaneous and intramuscular injections, such as glatiramer acetate, interferon beta and ofatumumab, advice on correct injection technique and rotation of the injection site will help reduce risk of injection site reactions (such as erythema, swelling, itching, pain). Advice should be drug/device specific and individual SPC should be consulted.
Mild to moderate infusion-related reactions (such as urticaria, pyrexia, dizziness, nausea, dyspnoea, flushing, rash, headache) can occur with alemtuzumab, ocrelizumab and natalizumab, normally during or within 24 hours of the infusion, and more often at the start of treatment. These can be managed with symptomatic treatment, pausing or slowing the infusion. With ocrelizumab and alemtuzumab, premedication with an antihistamine, corticosteroid and paracetamol are recommended. Regular monitoring of vital signs throughout the infusion and for a period after is also required. Occasionally, more severe hypersensitivity or anaphylactic reactions can occur that would warrant stopping treatment[63–65].
Infection
Owing to their mode of action, infections (e.g. respiratory or urinary tract infections) are more common with immunosuppressant DMTs (e.g. alemtuzumab, fingolimod, ocrelizumab); opportunistic infections occur rarely. The risk of progressive multifocal leukoencephalopathy (PML) — a brain infection caused by the John Cunningham virus (JCV) — with natalizumab is well established, this risk increases with duration of treatment, high anti-JCV antibody titre and prior immunosuppressant medication.
There is no known effective treatment for PML, management requires early detection by MRI scan and symptom recognition, as well as immediate discontinuation of the causative DMT, allowing the immune system to recover. Patients should be counselled that PML symptoms can include new or worsening neurological or psychiatric symptoms — such as altered vision, weakness, confusion, speech problems or personality changes — which can last for more than a few days. Patients should also be warned that features may be similar to an MS relapse[68].
There have been cases of PML associated with other DMTs including fingolimod, cladribine, dimethyl fumarate, ocrelizumab and alemtuzumab. For dimethyl fumarate, risk of PML is higher with prolonged lymphopenia. Regular MRI scans are recommended to monitor for PML where risk may be increased[8,61,64,68].
Herpes infections are associated with the monoclonal antibodies (natalizumab, alemtuzumab, ocrelizumab, ofatumumab), siponimod, fingolimod, ponesimod, dimethyl fumarate and cladribine. In these cases, prevention is important and, in specific cases (e.g. alemtuzumab and low lymphocyte levels on cladribine), antiviral prophylaxis may be used. Ocrelizumab and ofatumumab have been known to cause reduction in immunoglobulins (IgA, IgM and IgG). Hypogammaglobulinemia (reduced IgG) has been associated with an increased risk of infection, there is no evidence currently to suggest a threshold for stopping treatment, however, clinicians should consider the increased risk of infection and discuss with the patient[33–36,61–66].
If a patient develops infection while taking DMT, a clinical assessment of the severity of infection and the risk versus benefit of stopping or continuing the DMT is required. Additional consideration is needed if the infection is considered severe, related to the DMT or there is a history of recurrent infections.
Liver dysfunction
Severe liver dysfunction is rare, but mild–moderate changes in liver function tests (LFTs) may be seen with some DMTs (e.g. see Table 7)[33–36,53–65]. If LFTs are raised, a risk assessment for continuation with additional monitoring or stopping treatment should be made. If discontinuing medication, the patient should be monitored until the dysfunction resolves. Patients should be aware of and seek urgent medical attention if they develop signs and symptoms of liver dysfunction, including:
- Yellowing of skin or eyes;
- Dark urine;
- Abdominal pain;
- Unexplained bleeding or bruising.
Lymphopenia and blood dyscrasias
The development of lymphopenia is common and expected with some DMTs. Interpretation requires balancing expected changes versus levels that may result in adverse effects[32].
For example, dimethyl fumarate results in a reduction in lymphocytes by around 30%, on average. Over several clinical trials, 41% of patients developed lymphopenia — 38% of patients’ lymphopenia was mild to moderate (≥0.5–<0.91) and 2% was severe (<0.5). If prolonged for six months or more, severe lymphopenia would warrant discontinuation owing to increased risk of PML[61]. Fingolimod reduces peripheral lymphocyte count to 20–30% of baseline values, a lymphocyte count of <0.2 may increase risk of infection and warrant consideration of holding treatment[62]. Specific individual guidance is available in the SPC for each drug[33–36,53–65].
Blood dyscrasias can occur with some DMTs. For example, thrombocytopenia has been observed in patients on alemtuzumab and natalizumab. Patients should be counselled on signs and symptoms, which include rashes, oral ulceration, unusual bruising or bleeding. Neutropenia is a common side effect with ofatumumab and ocrelizumab, it is normally transient but should be investigated if infection develops [33–36,53–65].
Stopping and switching
The stopping criteria for DMTs include:
- Developing the inability to walk (EDSS score of >7), lasting more than six months (stop all DMTs);
- Development of secondary progressive disease with an observable increase in disability for more than a 12-month period, in the absence of clinical or radiological activity (stop all DMTs);
- Intolerance to the DMT (switch);
- Lack of efficacy, indicated by no reduction in frequency or severity of relapses compared to the pre-treatment phase, after taking the DMT for at least six months (switch)[6].
Patients can be switched between DMTs within the same eligibility group owing to intolerance. If the DMT is not effective, treatment should be escalated to a different DMT according to the criteria set out in Table 3. When switching, a washout period – a period of time where a drug is stopped prior to commencing a new therapy – is normally required. There is little evidence available to guide washout periods, which vary between DMTs.
Some information is available in the individual SPCs; however, in practice it is normally determined by balancing half-life of the DMT and duration of immunosuppression on stopping (to reduce risk of additive immunosuppression and adverse effects), against risk of a rebound relapse. The risk of rebound relapse is particularly high with natalizumab and fingolimod[69]. Interferon beta and glatiramer acetate are the only DMTs that do not require a washout period[53–59]. Switching or stopping DMTs can be difficult for patients as it means starting the treatment journey again. The MS team will provide support and counselling throughout to help the patient understand the reasons for switching and aid decision making.
Pregnancy, contraception and breastfeeding
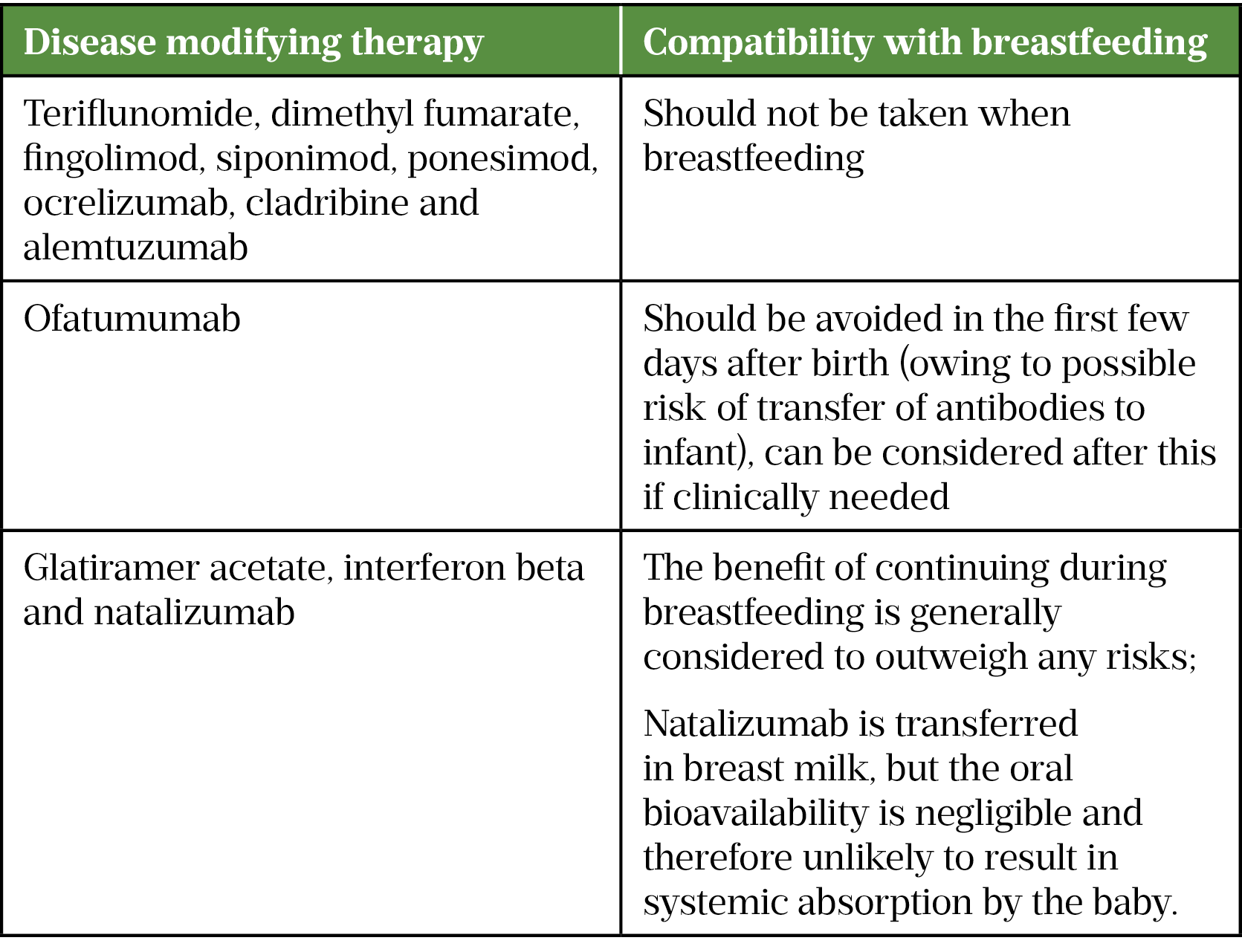
The use of DMTs should only be considered in pregnancy or breastfeeding where the benefits outweigh the risks. Prior to starting and at regular intervals (at least annually) throughout treatment, all women of child-bearing age (and men for cladribine) should be counselled on the associated risks, which vary between DMTs. Relapse rates may reduce during pregnancy but increase in the post-partum period.
If DMTs are stopped, a plan should be made to restart postpartum to minimise risk of relapsing. When stopping DMTs, a washout period may be required prior to trying to conceive[70].
Based on current available data from pregnancy registries, natalizumab (in the first and second trimester), interferon beta and glatiramer acetate (Copaxone) are considered relatively low risk in pregnancy[70]. There is good evidence to support the use of glatiramer acetate (Copaxone) in pregnancy, however, evidence for the Brabio brand is lacking, it should be noted that they are biosimilars so cannot be considered exactly the same[58,59,71].
There is insufficient data to determine whether dimethyl fumarate, ocrelizumab, ofatumumab or alemtuzumab are safe in pregnancy. There is a known or potential risk of teratogenicity associated with teriflunomide, fingolimod, cladribine, siponimod and ponesimod. Appropriate contraception is recommended for women of child-bearing potential on these medicines[33,34,36,70,72].
Vaccines
Historically, there has been some vaccine hesitancy in MS patients and clinicians owing to observations from case studies suggesting potential links between certain vaccines and MS. However, current evidence does not support a link between vaccines and developing MS or risk of relapse[73].
Patients on DMTs who are immunocompromised may be at an increased risk of vaccine preventable disease. Pharmacists can advocate, educate and advise both patients and other healthcare professionals on the use of vaccines in MS.
In patients who are immunosuppressed, live vaccines are contraindicated. Inactivated vaccines are safe but may evoke a reduced response[74]. There is limited evidence on the significance of this for most vaccines; however, emerging evidence from the COVID-19 vaccines indicate that this is more significant for certain DMTs, such as ocrelizumab and fingolimod[74–77]. Timing of vaccines in relation to DMT initiation is therefore important. It is preferable to complete vaccination before starting DMT; optimum timing for live vaccines is at least four to six weeks and for inactivated at least two to four weeks prior to commencement of treatment[74].
All patients with MS should be encouraged to receive the COVID-19 and annual influenza vaccines (which are inactivated). A single booster dose of the pneumococcal vaccine (PPV23) is also recommended for patients on long-term immunosuppression[64,65].
Where optimum timing is not possible (e.g. during a DMT switch where vaccinating will risk delay and rebound relapse, or if already on treatment), routine vaccination (if inactivated) should still be recommended as some protection is preferable.
Conclusion
Initiating and monitoring patients with MS on DMTs has become increasingly complex over the past ten years; throughout the patient treatment journey pharmacists can have a significant impact. Hospitals are starting to develop MS pharmacist roles that are vital to ensuring medicines optimisation for these patients. It is important that community and primary care pharmacists are aware of DMTs so that they can appropriately signpost patients presenting with side effects.
- 1Research and analysis, Multiple Sclerosis: prevalence, incidence and smoking status-data briefing. Public Health England. 2020.https://www.gov.uk/government/publications/multiple-sclerosis-prevalence-incidence-and-smoking-status/multiple-sclerosis-prevalence-incidence-and-smoking-status-data-briefing (accessed Mar 2022).
- 2National Institute for Health and Care Excellence. Multiple Sclerosis in adults: management. Clinical guideline (CG186). 2014.https://www.nice.org.uk/guidance/cg186 (accessed Mar 2022).
- 3Expanded Disability Status Scale (EDSS). Multiple Sclerosis Trust. 2020.https://mstrust.org.uk/a-z/expanded-disability-status-scale-edss (accessed Mar 2022).
- 4Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. The Lancet Neurology. 2018;17:162–73. doi:10.1016/s1474-4422(17)30470-2
- 5Giovannoni G, Butzkueven H, Dhib-Jalbut S, et al. Brain health: Time matters in multiple sclerosis. MS Brain Health. 2017.https://www.msbrainhealth.org/wp-content/uploads/2021/05/brain-health-time-matters-in-multiple-sclerosis-policy-report.pdf (accessed Mar 2022).
- 6Treatment Algorithm for Multiple Sclerosis Disease Modifying Therapies . NHS England. 2019.https://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2019/03/Treatment-Algorithm-for-Multiple-Sclerosis-Disease-Modifying-Therapies-08-03-2019-1.pdf (accessed Mar 2022).
- 7GITTO L. Living with Multiple Sclerosis in Europe: Pharmacological Treatments, Cost of Illness, and Health-Related Quality of Life Across Countries. Multiple Sclerosis: Perspectives in Treatment and Pathogenesis. 2017;:17–37. doi:10.15586/codon.multiplesclerosis.2017.ch2
- 8The Multiple Sclerosis Trust. The Multiple Sclerosis Trust. https://mstrust.org.uk/ (accessed Mar 2022).
- 9Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. The Lancet. 2012;380:1819–28. doi:10.1016/s0140-6736(12)61769-3
- 10Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. The Lancet. 2012;380:1829–39. doi:10.1016/s0140-6736(12)61768-1
- 11Giovannoni G, Comi G, Cook S, et al. A Placebo-Controlled Trial of Oral Cladribine for Relapsing Multiple Sclerosis. N Engl J Med. 2010;362:416–26. doi:10.1056/nejmoa0902533
- 12Polman CH, O’Connor PW, Havrdova E, et al. A Randomized, Placebo-Controlled Trial of Natalizumab for Relapsing Multiple Sclerosis. N Engl J Med. 2006;354:899–910. doi:10.1056/nejmoa044397
- 13Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N Engl J Med. 2017;376:221–34. doi:10.1056/nejmoa1601277
- 14Hauser SL, Bar-Or A, Cohen JA, et al. Ofatumumab versus Teriflunomide in Multiple Sclerosis. N Engl J Med. 2020;383:546–57. doi:10.1056/nejmoa1917246
- 15Gold R, Kappos L, Arnold DL, et al. Placebo-Controlled Phase 3 Study of Oral BG-12 for Relapsing Multiple Sclerosis. N Engl J Med. 2012;367:1098–107. doi:10.1056/nejmoa1114287
- 16Fox RJ, Miller DH, Phillips JT, et al. Placebo-Controlled Phase 3 Study of Oral BG-12 or Glatiramer in Multiple Sclerosis. N Engl J Med. 2012;367:1087–97. doi:10.1056/nejmoa1206328
- 17Kappos L, Radue E-W, O’Connor P, et al. A Placebo-Controlled Trial of Oral Fingolimod in Relapsing Multiple Sclerosis. N Engl J Med. 2010;362:387–401. doi:10.1056/nejmoa0909494
- 18Calabresi PA, Radue E-W, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. The Lancet Neurology. 2014;13:545–56. doi:10.1016/s1474-4422(14)70049-3
- 19Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: Results of a phase III multicenter, double-blind, placebo-controlled trial. Neurology. 1995;45:1268–76. doi:10.1212/wnl.45.7.1268
- 20Interferon beta-1b is effective in relapsing-remitting multiple sclerosis: I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43:655–655. doi:10.1212/wnl.43.4.655
- 21Ebers GC. Randomised double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. The Lancet. 1998;352:1498–504. doi:10.1016/s0140-6736(98)03334-0
- 22Confavreux C, O’Connor P, Comi G, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Neurology. 2014;13:247–56. doi:10.1016/s1474-4422(13)70308-9
- 23O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized Trial of Oral Teriflunomide for Relapsing Multiple Sclerosis. N Engl J Med. 2011;365:1293–303. doi:10.1056/nejmoa1014656
- 24Kappos L, Fox RJ, Burcklen M, et al. Ponesimod Compared With Teriflunomide in Patients With Relapsing Multiple Sclerosis in the Active-Comparator Phase 3 OPTIMUM Study. JAMA Neurol. 2021;78:558. doi:10.1001/jamaneurol.2021.0405
- 25Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N Engl J Med. 2017;376:209–20. doi:10.1056/nejmoa1606468
- 26Department of Error. The Lancet. 2018;392:2170. doi:10.1016/s0140-6736(18)32834-4
- 27Summary of product characteristics: Betaferon 250 microgram/ml, powder and solvent for solution for injection (beta interferon 1b) (Bayer plc). Medicines.org.uk. http://www.medicines.org.uk/emc/medicine/1809 (accessed Mar 2022).
- 28Summary of product characteristics: Extavia® (beta interferon 1b) 250 microgram/ml powder and solvent for solution for injection (Novartis Pharmaceuticals UK Ltd). Medicines.org.uk. http://www.medicines.org.uk/emc/medicine/21659 (accessed Mar 2022).
- 29Kappos L. Placebo-controlled multicentre randomised trial of interferon β-1b in treatment of secondary progressive multiple sclerosis. The Lancet. 1998;352:1491–7. doi:10.1016/s0140-6736(98)10039-9
- 30Interferon beta-1b in secondary progressive MS: Results from a 3-year controlled study. Neurology. 2004;63:1788–95. doi:10.1212/01.wnl.0000146958.77317.3e
- 31Palace J, Duddy M, Lawton M, et al. Assessing the long-term effectiveness of interferon-beta and glatiramer acetate in multiple sclerosis: final 10-year results from the UK multiple sclerosis risk-sharing scheme. J Neurol Neurosurg Psychiatry. 2018;90:251–60. doi:10.1136/jnnp-2018-318360
- 32Fox EJ, Buckle GJ, Singer B, et al. Lymphopenia and DMTs for relapsing forms of MS. Neurol Clin Pract. 2019;9:53–63. doi:10.1212/cpj.0000000000000567
- 33Summary of product characteristics. Mayzent 2mg film coated tablets (Novartis Pharmaceuticals UK Ltd). Medicines.org.uk. https://www.medicines.org.uk/emc/product/11020/smpc (accessed Mar 2022).
- 34Summary of product characterisitcs. Kesimpta 20mg solution for injection in pre-filled pen (Novartis Pharmaceuticals UK Ltd). Medicines.org.uk. https://www.medicines.org.uk/emc/product/12433 (accessed Mar 2022).
- 35Summary of product characteristics. Cladribine (Mavenclad) 10mg tablets (Merk). Medicines.org.uk. https://www.medicines.org.uk/emc/product/8435 (accessed Mar 2022).
- 36Summary of product characteristics. Ponvory 20mg film coated tablets (Janssen-Cilag Ltd). Medicines.org.uk. https://www.medicines.org.uk/emc/product/12798/smpc (accessed Mar 2022).
- 37Hannan G, Sopala J, Roberts M. MS Specialist Nursing in the UK 2018: Results from the 2018 MSTrust Nurse Mapping Survey. The MS Trust. 2018.https://mstrust.org.uk/sites/default/files/Nurse%20Mapping%202018%20WEB.pdf (accessed Mar 2022).
- 38Nitkunan A, Lawrence J, Reilly MM. Neurology Workforce Survey conducted by the Association of British Neurologists. https://cdn.ymaws.com/www.theabn.org/resource/collection/219B4A48-4D25-4726-97AA-0EB6090769BE/2020_ABN_Neurology_Workforce_Survey_2018-19_28_Jan_2020.pdf. 2020.https://cdn.ymaws.com/www.theabn.org/resource/collection/219B4A48-4D25-4726-97AA-0EB6090769BE/2020_ABN_Neurology_Workforce_Survey_2018-19_28_Jan_2020.pdf (accessed Mar 2022).
- 39Dimethyl fumarate for treating relapsing-remitting multiple sclerosis. Technology appraisal 320. National Institute for Health and Care Excellence. 2014.https://www.nice.org.uk/guidance/ta320 (accessed Mar 2022).
- 40Teriflunomide for treating relapsing-remitting multiple sclerosis. Technology appraisal 303. National Institute for Health and Care Excellence. 2014.https://www.nice.org.uk/guidance/ta303 (accessed Mar 2022).
- 41Fingolimod for the treatment of highly active relapsing-remitting multiple sclerosis. Technology appraisal 254. National Insitute for Health and Care Excellence . 2012.https://www.nice.org.uk/guidance/ta254 (accessed Mar 2022).
- 42Alemtuzumab for treating relapsing-remitting multiple sclerosis. Technology appraisal 312. National Institute for Health and Care Excellence. 2014.https://www.nice.org.uk/guidance/ta312 (accessed Mar 2022).
- 43Natalizumab for the treatment of adults with highly active relapsing-remitting multiple sclerosis. National Institute for Health and Care Excellence. 2007.https://www.nice.org.uk/guidance/ta127 (accessed Mar 2022).
- 44Cladribine for treating relapsing-remitting multiple sclerosis. Technology appraisal 616. National Institute for Health and Care Excellence. 2018.https://www.nice.org.uk/guidance/ta616 (accessed Mar 2022).
- 45Ocrelizumab for treating relapsing-remitting multiple sclerosis. Technology appraisal 533. National Institute for Health and Care Excellence. 2018.https://www.nice.org.uk/guidance/ta533 (accessed Mar 2022).
- 46Beta interferons and glatiramer acetate for treating multiple sclerosis. Technology appraisal TA527. National Institute for Health and Care Excellence. 2018.https://www.nice.org.uk/guidance/ta527 (accessed Mar 2022).
- 47Siponimod for treating secondary progressive multiple sclerosis. Technology appraisal TA656. National Institute for Health and Care Excellence. 2020.https://www.nice.org.uk/guidance/ta656 (accessed Mar 2022).
- 48Ofatumumab for treating relapsing multiple sclerosis. Technology appraisal TA699. National Institute for Health and Care Excellence. 2021.https://www.nice.org.uk/guidance/ta699 (accessed Mar 2022).
- 49Ocrelizumab for treating primary progressive multiple sclerosis. Technology appraisal TA585. National Institute for Health and Care Excellence. 2019.https://www.nice.org.uk/guidance/ta585 (accessed Mar 2022).
- 50Ponesimod for treating relapsing-remitting multiple sclerosis. Final appraisal document. National Institute for Health and Care Excellence. 2021.https://www.nice.org.uk/guidance/gid-ta10556/documents/final-appraisal-determination-document (accessed Mar 2022).
- 51Ponesimod for treating relapsing–remitting multiple sclerosis. National Institute for Health and Care Excellence. https://www.nice.org.uk/guidance/ta767 (accessed Mar 2022).
- 52The MS Society. The MS Society. https://www.mssociety.org.uk (accessed Mar 2022).
- 53Summary of product characteristics: Rebif (beta interferon 1a) Solution for injection pre-filled pens (Merck Serono). Medicines.org.uk. http://www.medicines.org.uk/emc/medicine/23881 (accessed Mar 2022).
- 54Summary of product characteristics: Betaferon 250 microgram/ml, powder and solvent for solution for injection (beta interferon 1b) (Bayer plc). Medicines.org.uk. http://www.medicines.org.uk/emc/medicine/1809 (accessed Mar 2022).
- 55Summary of product characteristics: Extavia® (beta interferon 1b) 250 microgram/ml powder and solvent for solution for injection (Novartis Pharmaceuticals UK Ltd). Medicines.org.uk. http://www.medicines.org.uk/emc/medicine/21659 (accessed Mar 2022).
- 56Summary of product characteristics: Plegridy 125microgram solution for injection in pre-filled pen (Biogen Idec Ltd). Medicines.org.uk. https://www.medicines.org.uk/emc/medicine/29370 (accessed Mar 2022).
- 57Summary of product characteristics: AVONEX (beta interferon 1a) 30 micrograms/0.5ml solution for injection, in pre-filled pen (Biogen Idec Ltd). Medicines.org.uk. http://www.medicines.org.uk/emc/medicine/24676 (accessed Mar 2022).
- 58Summary of product characteristics: Copaxone (Glatiramer acetate) 20mg/ml, solution for injection, pre-filled syringe (Teva Pharmaceuticals Ltd). Medicines.org.uk. http://www.medicines.org.uk/emc/medicine/17516 (accessed Mar 2022).
- 59Summary of product characteristics: Copaxone (Glatiramer acetate) 40mg/ml, solution for injection, pre-filled syringe (Teva Pharmaceuticals Ltd). Medicines.org.uk. http://www.medicines.org.uk/emc/medicine/30795 (accessed Mar 2022).
- 60Summary of product characteristics. Aubagio (Teriflunomide) 14mg film coated tablets (Genzyme therapeutics). Medicines.org.uk. https://www.medicines.org.uk/emc/medicine/28533 (accessed Mar 2022).
- 61Tecfidera 240mg gastro-resistant hard capsules (Biogen Idec Ltd). Medicines.org.uk. https://www.medicines.org.uk/emc/medicine/28593 (accessed Mar 2022).
- 62Summary of product characteristics. Gilenya (Fingolimod) 0.5mg hard capsules (Novartis Pharmaceuticals UK Ltd). Medicines.org.uk. http://www.medicines.org.uk/emc/medicine/24443 (accessed Mar 2022).
- 63Summary of product characteristics. Lemtrada 12mg concentrate for solution for infusion (Genzyme Therapeutics). Medicines.org.uk. https://www.medicines.org.uk/emc/medicine/28917 (accessed Mar 2022).
- 64Summary of Product Characteristics. Tysabri 300mg concentrate for solution for infusion (Biogen Idec ltd). Medicines.org.uk. https://www.medicines.org.uk/emc/medicine/18447 (accessed Mar 2022).
- 65Summary of product characteristics. Ocrelizumab (Ocrevus) 300mg concentrate for solution for infusion. Medicines.org.uk. https://www.medicines.org.uk/emc/product/8898/smpc (accessed Mar 2022).
- 66Moiola L, Barcella V, Benatti S, et al. The risk of infection in patients with multiple sclerosis treated with disease-modifying therapies: A Delphi consensus statement. Mult Scler. 2020;27:331–46. doi:10.1177/1352458520952311
- 67Disease modifying drugs (DMDs). MS Trust . 2021.https://mstrust.org.uk/about-ms/ms-treatments/disease-modifying-drugs-dmds (accessed Mar 2022).
- 68Natalizumab (Tysabri): progressive multifocal leukoencephalopathy-updated advice to support early detection. Medicines and Healthcare Products Regulatory Agency. 2016.https://www.gov.uk/drug-safety-update/natalizumab-tysabri-progressive-multifocal-leukoencephalopathy-updated-advice-to-support-early-detection (accessed Mar 2022).
- 69Barry B, Erwin AA, Stevens J, et al. Fingolimod Rebound: A Review of the Clinical Experience and Management Considerations. Neurol Ther. 2019;8:241–50. doi:10.1007/s40120-019-00160-9
- 70Dobson R, Dassan P, Roberts M, et al. UK consensus on pregnancy in multiple sclerosis: ‘Association of British Neurologists’ guidelines. Pract Neurol. 2019;19:106–14. doi:10.1136/practneurol-2018-002060
- 71Brabio 40 mg/mL Solution for Injection, Pre-filled Syringe. Medicines.org.uk. https://www.medicines.org.uk/emc/product/9181/smpc
- 72Medicines with teratogenic potential: what is effective contraception and how often is pregnancy testing needed? Medicines and Healthcare products Regulatory Agency. 2019.https://www.gov.uk/drug-safety-update/medicines-with-teratogenic-potential-what-is-effective-contraception-and-how-often-is-pregnancy-testing-needed (accessed Mar 2022).
- 73Reyes S, Ramsay M, Ladhani S, et al. Protecting people with multiple sclerosis through vaccination. Pract Neurol. 2020;20:435.1-445. doi:10.1136/practneurol-2020-002527
- 74Immunisation against infectious disease. UK Health Security Agency. 2013.https://www.gov.uk/government/collections/immunisation-against-infectious-disease-the-green-book (accessed Mar 2022).
- 75Sormani MP, Inglese M, Schiavetti I, et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. eBioMedicine. 2021;72:103581. doi:10.1016/j.ebiom.2021.103581
- 76ABN MS and Neuroinflammation Advisory Group. ABN GUIDANCE ON THE USE OF DISEASE-MODIFYING THERAPIES IN MULTIPLE SCLEROSIS IN RESPONSE TO THE COVID19 PANDEMIC. Association of British Neurologists (ABN). 2021.https://cdn.ymaws.com/www.theabn.org/resource/collection/6750BAE6-4CBC-4DDB-A684-116E03BFE634/21.10.26_ABN_Guidance_on_DMTs_for_MS_and_COVID-19.pdf (accessed Mar 2022).
- 77MS Society Medical Advisers consensus statement on MS treatments and COVID-19 vaccines. . MS Society UK. 2022.https://www.mssociety.org.uk/what-we-do/news/ms-society-medical-advisers-release-consensus-statement-covid-19-vaccines (accessed Mar 2022).