Shutterstock.com
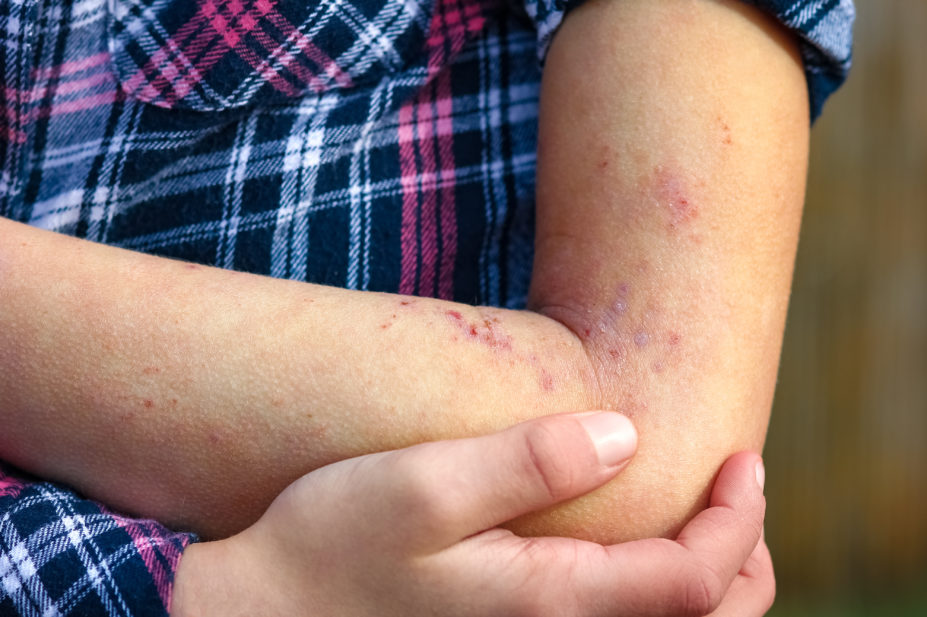
A biologic drug has been shown to reduce eczema symptoms in infants and young children by 75% in what is thought to be the first study of a monoclonal antibody for the treatment of skin conditions in children.
The study, led by Northwestern Medicine in Chicago, found that a monoclonal antibody, known as dupilumab, is “highly effective” in reducing signs of eczema and itching in children aged six months to five years.
It will offer an alternative to immune-suppression medication, the researchers said, in their findings published in The Lancet.
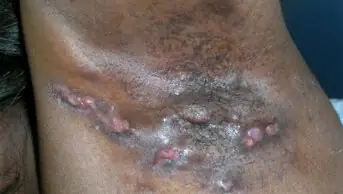
An estimated 19% of children aged under six years have eczema, and for up to 90% of individuals who have it, eczema starts during the first five years of life, the researchers said.
Most young children can be treated with steroid ointment and moisturisers. However, in one-third of cases seen in young children, the itch can be debilitating, causing sleep disturbance and poor neurocognitive development. In these cases, more aggressive treatments are required.
During the study, children with moderate to severe eczema were treated over 16 weeks with dupilumab, a medication that targets a critical immune pathway in allergies. The data were collected at 31 sites across Europe and North America.
More than half of the children in the study had at least a 75% reduction in signs of eczema, as well as reductions in itch and improved sleeping patterns.
Amy Paller, lead study author from Northwestern University and a dermatologist at the Lurie Children’s Hospital in Chicago, said: “Atopic dermatitis or eczema is so much more than just itchy skin. It is a devastating disease. The quality of life of severe eczema — not only for the child but also parents — is equivalent to many life-threatening diseases.
“The effect for most of these younger children is dramatic and at least as good as we’ve seen with the risky immunosuppressant medications.”
Commenting on the findings, Dan Hawcutt, senior lecturer paediatric pharmacology at the University of Liverpool and consultant paediatric clinical pharmacologist at Alder Hey Children’s Hospital, said: “The clinical need for improved treatments for the youngest children is clear, as eczema is very common in this age group. Oral steroids, in particular, have unpleasant adverse effects, both physical, biochemical and neuropsychological.”
He explained that paediatric dermatologists already use the drug in clinical practice since it is licensed in the UK for older children (aged six years and over) with the most severe eczema.
But he added: “It is very exciting to see data being presented for the youngest children for dupilumab in eczema.”
Hawcutt cautioned that additional long-term assessment of the risks and benefits of dupilumab in this new younger population would be required since the study compared the treatment with dupilumab against placebo and not current standard of care. “As with all new drugs, new possible harms may be identified through spontaneous reporting schemes like MHRA Yellow Card,” he said.