Shutterstock.com
The Medicines and Healthcare products Regulatory Agency (MHRA) has authorised the use of tirzepatide (Mounjaro; Eli Lilly) for weight loss and weight management in adults.
In a statement published on 8 November 2023, the MHRA announced that adults with obesity (a body mass index [BMI] of ≥30kg/m²) or who are overweight (BMI between 27–30kg/m²) with weight-related health problems — such as prediabetes, high blood pressure, high cholesterol, or heart problems — will be able to receive the subcutaneous injection.
The announcement follows positive results from two international randomised controlled trials, SURMOUNT-1 and SURMOUNT-2, which included overweight and obese adult patients, with and without diabetes. Results of the studies showed that patients who were treated with tirzepatide had significant weight loss over time compared with patients who took a placebo.
In September 2023, an early publication of a meta-analysis study abstract also found that tirzepatide was more effective in reducing body weight than semaglutide — which was recommended as a weight loss treatment for NHS use in England by the National Institute for Health and Care Excellence (NICE) in March 2023 — at all respective doses.
Tirzepatide is a glucagon-like peptide (GLP-1) receptor agonist, which is injected under the skin of a patient’s stomach area, thigh or upper arm once per week. It works by regulating patient’s appetite, making them feel less hungry and experience fewer food cravings.
In its statement, the MHRA said the treatment is to be used alongside a reduced-calorie diet and increased physical activity.
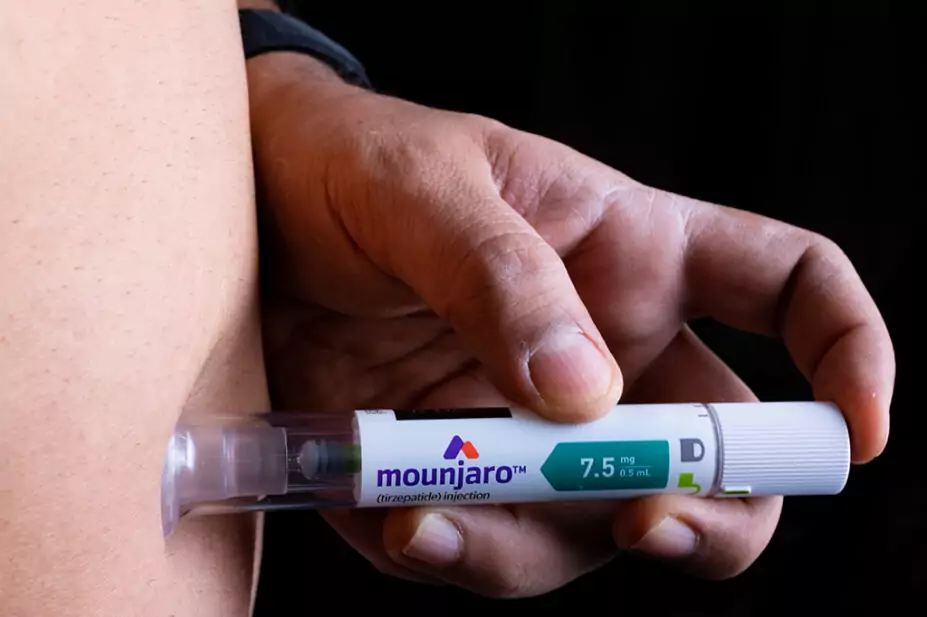
Mounjaro is available for weight management as a pre-filled injection pen, filled with 2.5mg, 5mg, 7.5mg, 10mg, 12.5mg or 15mg of tirzepatide. The starting dose is 2.5mg once per week for four weeks, increasing to 5mg once per week. The dose may then be increased in at least 4-week intervals up to the maximum dose of 15mg once weekly, if recommended by a doctor.
Commenting on the approval, Julian Beach, interim executive director of the MHRA, said: “We have prioritised rapid assessment of this new indication for Mounjaro, given the public health importance of access to new medicines to help tackle obesity.
“We have drawn on advice from the independent Commission on Human Medicines in coming to our decision, and as with all products, will keep the safety of Mounjaro under close review.”
In October 2023, tirzepatide was recommended by the National Institute for Health and Care Excellence (NICE) for the treatment of type 2 diabetes mellitus, reversing its initial decision against use of the drug in June 2023.
In its final draft guidance, published on 8 September 2023, NICE said its independent committee was able to make the positive recommendation on the new treatment following “additional analyses and modelling on clinical and cost effectiveness” provided by the manufacturer after the initial consultation.
In March 2023, semaglutide (Wegovy; Novo Nordisk) was approved for NHS treatment for weight loss by NICE. In September 2023, manufacturer Novo Nordisk said the product would be made available for prescription from 4 September 2023 as part of a “controlled and limited launch” of the drug in the UK.