Abstract
The current recommended treatment for portal vein thrombosis (PVT) is low molecular weight heparins (LMWH) or oral vitamin K antagonists, such as warfarin, for at least three or six months. There has been growing interest among clinicians within our tertiary referral centre for liver disease at King’s College Hospital (KCH) to prescribe direct oral anticoagulants (DOACs) for PVT. There is a lack of trial data and guidelines available for this indication; therefore, a comprehensive therapeutic review was conducted to inform best evidence-based practice.
A literature search was conducted using the Healthcare Databases Advanced Search (HDAS) platform for Medline and EMBASE and the results screened for eligibility, resulting in ten papers to be included for evaluation and discussion. The literature analysed in this review suggests that DOACs may be a safe and effective alternative for PVT; however, it must be noted there is still limited quality evidence and patient variability remains broad, which makes universal recommendations difficult.
Rivaroxaban is cited in the literature as being a potential effective anticoagulant choice in PVT. An individualised and patient-centred approach must be undertaken when assessing the suitability of prescribing DOACs in patients with PVT.
Introduction
King’s College Hospital (KCH) is a tertiary referral centre for hepatology and has the largest transplantation programme in Europe, carrying out more than 200 procedures annually. It is also a specialist centre for hepato-pancreato-biliary surgery, conducting up to 400 Whipples and splenectomy procedures per year. Complications in the post-operative period can result in prolonged patient admission and possible poorer patient outcomes, including portal vein thrombosis (PVT). In the general population, incidence of PVT has been shown to be between 0.05 and 0.50% in post-mortem studies. However, this incidence rises in those with liver disease, such as in people with cirrhosis[1].
Acute PVT is defined as the formation of a sudden clot in the hepatic portal vein that leads to damage to the vessel wall, hypercoagulability and reduced blood flow, resulting in obstruction and portal hypertension[2–4]. Abdominal pain is present in 90% of acute PVT cases and ascites is visible in around 50% of patients when diagnosed using non-invasive imaging methods[5]. PVT can extend into the mesenteric or splenic veins and occlusions can be diagnosed as complete or partial[2]. This occlusion consequently causes a deterioration in liver function and mortality. Intestinal infarction is a severe complication for patients in which the thrombus extends to the superior mesenteric vein[3]. Patients with non-cirrhotic acute PVT may first be triaged to general surgical wards because the primary symptom is abdominal pain; however, management of PVT in cirrhotic patients may be restricted to gastrointestinal and hepatology wards[5].
Chronic PVT develops in patients with acute PVT that does not resolve, with or without treatment[2–4]. There is cavernous transformation of the portal vein, characterised by the development of collateral blood vessels around the area of obstruction, which can be identified on CT scans. Complications of chronic PVT include portal hypertension and portal cholangiopath[2–4].
Risk factors for PVT include lethargic portal blood flow secondary to cirrhosis, comorbidities including diverticulitis, inflammatory bowel disease, cholangitis, haematological malignancies or JAK2 mutations[2,4]. The formation of a PVT can be further instigated in the post-operative period, for example, trauma following a portosystemic shunt, liver transplantation, splenectomy or localised-regional treatments, such as transcatheter arterial chemoembolisation for hepatocellular carcinoma[2–4].
Current guidelines on the pharmacological management of PVT — based on the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) guidelines — recommend the use of low molecular weight heparins (LMWHs) for at least three to six months for treatment of acute PVT[2,3]. Permanent anticoagulation is recommended in patients with established prothrombotic disease[3]. LMWH can be switched to oral vitamin K antagonists, such as warfarin, with a target international normalised ratio (INR) of 2.0–3.0[2]. A target INR is necessary to ensure warfarin provides anticoagulation benefit while avoiding the risk of haemorrhage[2]. Treatment aims to prevent further thrombi extension and achieve recanalisation of the PVT[2,3]. Outcomes are measured using a CT scan to assess degree of recanalisation of the portal venous system at 6 to 12-month follow up[2].
Direct oral anticoagulants (DOACs), including rivaroxaban, edoxaban and dabigatran, are unlicensed for PVT treatment; however, these factor Xa and direct thrombin inhibitors offer important pharmacological benefits over oral vitamin K antagonists and LMWHs. The rapid onset and offset of action, fixed dosing, fewer drug–drug interactions, absence of regular coagulation monitoring and avoiding the need for daily injections make them of interest to both prescribers and patients[6]. Rivaroxaban has been initiated for PVT in patients with or without cirrhosis at KCH based on consultant decision.
As per the manufacturer guidance, all DOACs currently available on the market are contraindicated in hepatic disease associated with coagulopathy and clinically significant bleeding risk[7–10]. This includes patients with cirrhosis with a Child-Pugh score of B or C. As a specialist centre for hepatology disease, KCH provides care for a variety of patients with varying degrees of liver failure. It is routine practice to treat patients with a severe degree of dysfunction of the cirrhotic liver, including those awaiting transplant.
There is a lack of evidence to support the use of DOACs for PVT and there are currently no NHS trust guidelines in this therapeutic area. Owing to the potential benefits offered by DOACs, it is pivotal a literature search is conducted and findings are critically evaluated to issue guidance for healthcare professionals on the use of these agents within the liver unit at KCH.
Literature search
On 30 December 2020, a literature search was conducted using the Healthcare Databases Advanced Search (HDAS) platform for Medline and EMBASE. Owing to the limited evidence and lack of clinical trials using DOACs in PVT, the search was conducted using broad terms, such as DOAC drug name, class and disease term. This returned many papers, so the search was then narrowed using specific search terms (such as ‘rivaroxaban’ and ‘factor Xa inhibitors’). A full list of search terms can be found in supplementary information. This generated 12 results on Medline and 37 results on EMBASE. These results were then further narrowed using the exclusion criteria (see Figure).
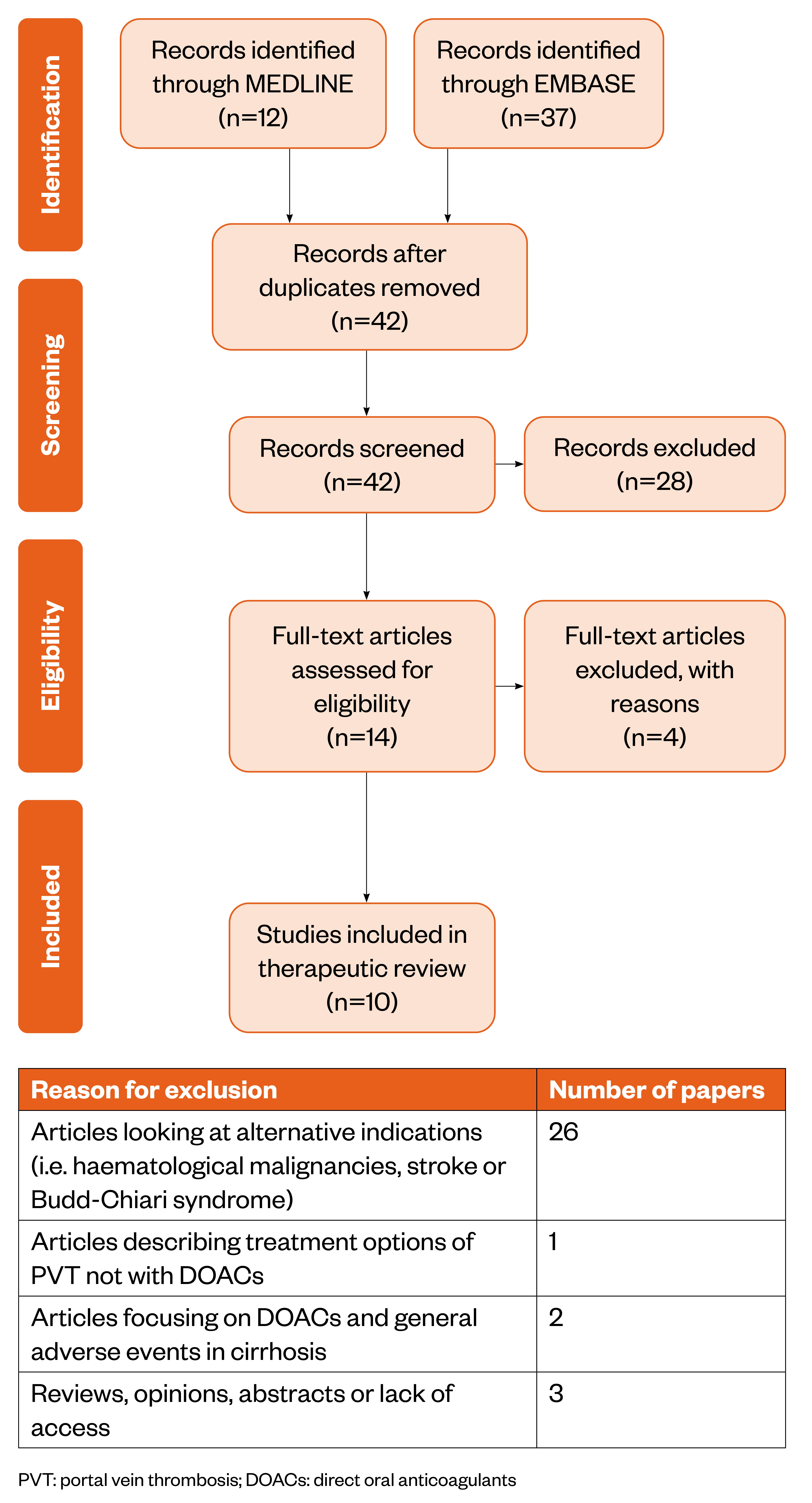
Guidelines
On 6 August 2018, a comprehensive search for guidelines on the management of PVT using DOACs was undertaken. Specialist centres in the UK known for treating liver disease, such as St Mary’s Hospital, Queen Elizabeth Hospital, Royal Infirmary of Edinburgh, Royal Free Hospital and St James’s University Hospital, were contacted but none had guidelines in this therapeutic area. There were no internal guidelines at KCH and none were found on the National Institute for Health and Care Excellence (NICE) website. EASL and AASLD are the only organisations that provide guidelines for the management of PVT; however, they do not describe DOACs as a treatment option[2,3].
Critical evaluation of the literature
Excluding case reports (n=6), the papers were independently critically analysed using the critical appraisals skills programme (CASP) toolkit (see Table 1: Evaluation of studies using the critical appraisals skills programme (CASP) toolkit)[11–15]. The following sections, grouped by study type, describe each of the identified papers.
Retrospective cohort studies
Nagaoki et al. present a retrospective cohort study with 50 patients that aimed to compare the efficacy and safety of edoxaban, administered after danaparoid (a LMWH) for two weeks, compared with warfarin[12]. There were 15 patients with Child-Pugh A cirrhosis and five patients with a Child-Pugh B cirrhosis in the edoxaban cohort. No patients with Child-Pugh C cirrhosis were considered owing to a high risk of bleeding. The duration of treatment for PVT was six months. The study examined the volume of PVT in which complete response was defined as complete resolution of the thrombus. The authors reported no significant distinction in the clinical features of patients of the two cohorts of patients with cirrhosis. There was a reduction in PVT with edoxaban from 1.42 cm3 at two weeks to 0.42cm3 at six months. Evidently, this is not complete resolution of thrombi, but there was a contrast when compared with those in the warfarin group, where an increase in PVT volume from 1.73 cm3 at two weeks to 2.85cm3 was observed. It should be noted that the target INR in the warfarin group was 1.5–2.0, which is subtherapeutic; the reason for this is unclear[12].
A total of 50 patients were split into an edoxaban (n=20) arm and a warfarin (n=30) arm. The rationale for the unequal division is not clear in the methodology, nor does the article clearly define the process of treatment allocation. The investigators included five patients with a Child-Pugh grade C in the warfarin arm but none in the edoxaban arm. This presents a disparity in patient characteristics, contrary to the investigators’ result. This is predictable in a study of this design, as it is difficult to ensure variables are standardised in the investigational cohort versus the control group. Follow up for both cohorts was only up to six months of treatment and there was no follow up post-termination of the DOAC. Therefore, it is difficult to conclude there was no recurrent PVT[12].
Naymagon et al. present a retrospective cohort study investigating the efficacy and safety of DOACs in patients with non-cirrhotic PVT[13]. There were 330 patients enrolled, receiving warfarin (n=108), enoxaparin (n=70), rivaroxaban (n=65), apixaban (n=20), dabigatran (n=8), fondaparinux (n=2) or no anticoagulant (n=57) with a follow up of at least three months.
The primary outcome measured was the rate of complete radiographic resolution (CRR) of PVT established on follow-up imaging. The authors state the presence of PVT in each case was confirmed by radiology report at diagnosis and any changes over time were assessed by reviewing subsequent reports. The authors reported DOACs were associated with the highest CRR rates (dabigatran [75%; n=6]; apixaban [65%; n=13]; rivaroxaban [65%; n=42]) in comparison to warfarin, which was associated with worse outcomes (CRR rate 31%; n=33). Enoxaparin, a LMWH, was associated with a CRR rate like that of DOACs. The outcomes of the fondaparinux group are not clear owing to the small sample size. Interestingly, only 62% of patients receiving warfarin achieved an INR within the desired therapeutic range of 2.0–3.0. The authors do not specify the percentage in which warfarin was subtherapeutic or supratherapeutic, this may have influenced the findings seen in the warfarin cohort. Furthermore, drug doses in all treatment groups are not clearly defined. The mean duration of follow up varied across the groups, with longest noted in those receiving warfarin (55.8 months) in comparison to the DOAC group (28.1 months); therefore, the potential for follow up bias cannot be overlooked[13].
Prospective cohort study
Ai et al. present a prospective cohort study investigating the efficacy and safety of DOACs in chronic PVT in cirrhotic patients (n=80), receiving rivaroxaban (n=26), dabigatran (n=14) or no anticoagulant (n=40), with follow up at three and six months[14]. They reported that after three months of treatment with a DOAC, complete recanalisation of PVT was achieved in 5.1% (n=2) of patients and partial recanalisation of PVT was observed in 7.7% (n=3) of patients. In contrast, the control group showed no change in PVT. At six months, complete recanalisation of PVT increased to 10.3% (n=4) in patients treated with a DOAC, while partial recanalisation increased to 17.9% (n=7) in patients treated with a DOAC and 2.6% (n=1) in the control group. DOAC results are presented as collective and the authors do not specify individual outcomes, making it difficult to determine if one DOAC was superior. Both the DOAC and control groups showed mild differences in median portal vein blood flow velocity at three months. The data at six months reported an improvement to 15.6cm/s in the DOAC group and 13.6cm/s in the control group. This was a statistically significant difference (P<0.05), indicating that treatment with DOACs for six months can further reduce the size of the chronic PVT and increase portal flow rate[14].
Child-Pugh classifications of B or C are contraindications for rivaroxaban and thus patients with these classifications were excluded from the rivaroxaban arm and instead allocated dabigatran[9,14]. The possibility of selection bias must be considered.
Randomised, controlled open label trial
Hanafy et al. conducted a randomised, controlled interventional open label study comparing the outcomes of rivaroxaban (n=40), with a control arm receiving warfarin (n=40), in people with cirrhosis receiving treatment for acute PVT from May 2014 to August 2017[15]. The primary outcome was complete recanalisation of the portal vein, or partial recanalisation, if reopening is 50% greater than the luminal occlusion. The secondary outcome measured was the absence of recurrent PVT following termination of therapy[15].
Rivaroxaban, at a dose of 10mg twice a day for six months, resulted in complete resolution of PVT and recanalisation in 85% of patients (n=34). Patients in the rivaroxaban cohort had no recurrent PVT, following termination of treatment[15].
The follow up period of this study is clearly defined. It must be noted that patients with a Child-Pugh classification of C, concomitant use of anticoagulants or antiplatelets and creatinine clearance <30mL/min (indicative of increased bleed risk) were excluded from the study. Thus, showing an attempt to standardise patient characteristics and limit known adverse effects in both arms; however, the location or expansion of thrombi in the portal vein was not described in selection process. Consequently, there may be disparities, in degree of occlusion size and if thrombi had expanded (e.g. into the mesenteric or splenic vein) within cohorts[15].
The methodology states that 80 patients who had undergone splenectomy owing to hypersplenism and four with acute PVT owing to portal pyaemia, were selected; however, the results only describe 80 individuals. Furthermore, process of treatment allocation was randomised, but it does not state how this randomisation occurred. The study design is open label and therefore introduces the possibility of selection bias[15].
Case reports
The six case reports analysed in this study are summarised in ‘Table 2: Summary and comparison of case reports‘[16–21]. The DOAC discussed in all cases is rivaroxaban. The cases depict differences in age, gender and past medical history. Subsequently, standardising outcomes or predicting response is not possible owing to differences in patient background.
Five reports share successful resolution of PVT; however, one details occurrence of PVT despite the patient being anticoagulated with rivaroxaban for previously established atrial fibrillation[16–21]. Interestingly, this patient had Child-Pugh B cirrhosis whereas the five exhibiting successful resolution had Child-Pugh A cirrhosis. Only one patient, a 28-year-old-female, was non-cirrhotic[18]. Authors reporting successful recanalisation with rivaroxaban, report no recurrence of PVT or adverse events, such as bleeding, in the varied follow-up periods ranging from one month to five months[16–20]. The therapeutic dose was 20mg daily in four cases, two of which initiated the patient with rivaroxaban 15mg twice daily for three weeks and then switched to 20mg once daily[16–18,20].
Lenz et al. report a 63-year-old female, without prior history of thrombotic disease, presenting with a partial PVT. She was treated with rivaroxaban 10mg daily for five months achieving complete resolution of thrombus; however, upon termination of DOAC, there was recurrence of PVT noted, highlighting that this dose may not be therapeutic[19]. It must be noted the treatment duration and response to DOAC varied in each case report and can be found in Table 2.
Discussion
Although DOACs offer a favourable alternative to currently recommended therapy, the long-term safety and efficacy data is not yet readily available. The short half-life of these drugs results in a rapid reduction in antithrombotic effect if a dose is missed[5,6]. Limited monitoring parameters cause difficulty in assessing patient compliance and doses must be adjusted based on renal function and age[5,6]. The pharmacokinetic profile of DOACs in cirrhotic liver disease can be erratic, impairing drug clearance as a result and leading to accumulation, which can increase adverse effects such as bleeding[5,6]. In addition, reversal agents for DOACs are costly and not widely available. Idarucizumab, an antidote for dabigatran, is available at around £2,400 per 5g dose[22]. The administration of a second 5g dose may be considered, for example, if there is recurrent clinically relevant bleeding alongside prolonged clotting times[22]. Andaxanet alfa, a licensed antidote for rivaroxaban and apixaban, is awaiting NICE approval and is priced at around £15,000 per patient[23].
Nagaoki et al. described adverse effects, including rectal varices, angiodysplasia of the small intestine and colon polyps in three patients; all treated with the lower dose of 30mg once daily[12]. However, there is insufficient data to indicate if the lower dose of edoxaban was as equally effective in reducing PVT size because the manufacturer’s recommended dose is 60mg daily [12]. The authors reported a complete response in 70% (n=14) of the edoxaban group at six months, but response level in the remaining 30% is not clearly discussed[12]. The study is retrospective therefore limited by its design and a larger sample size is necessary to draw conclusions. Naymagon et al. enrolled a larger sample size (n=330); however, patients with cirrhosis were excluded[13]. The study did not define the doses used for each treatment intervention, therefore correlating results to a particular dose is difficult. The follow-up period varies across the groups and adverse events are reported collectively rather than for an individual drug, impeding further analysis.
Ai et al. report outcomes in chronic PVT only; therefore, results are not directly applicable to patients with acute PVT[15]. The bleeding events reported were low; however, the study does not list all patient factors considered for treatment allocation. The sample size is small (n=80) and further follow up post six months would be needed to evaluate long-term efficacy and safety[15].
Hanafy et al. investigated rivaroxaban as the agent of choice[14]. Exclusion criteria included patients taking antiplatelet or anticoagulant drugs; however, the patient population seen at KCH are more likely to be taking an antiplatelet or co-prescribed following a splenectomy procedure if platelets are elevated, therefore limiting comparison with this cohort[14,24]. Nonetheless, Hanafy et al. describe a clear follow-up period and no recurrent PVT was observed in their patients[14].
The six case reports included in the review investigated rivaroxaban as the DOAC of choice[16–21]. Limitations with these reports included the significant diversity in patient characteristics and lack of reporting on initial treatment choice, dose of rivaroxaban selected and location of PVT, which make the comparison between studies challenging. The case reports do not describe degree of renal impairment — while patients with severe renal impairment were excluded from the other studies described in this review — thus restricting our understanding of DOACs in this subclass of patients who may present with PVT.
There is a lack of randomised controlled trials, except one, limiting the quality of available data. Retrospective cohort, prospective cohort or case reports are not controlled studies; factors impacting anticoagulant prescribing may not be under the investigator’s control. Additionally, there is inadequate data available to support the use of DOACs in patients classified as Child-Pugh C.
There were seven articles primarily assessing rivaroxaban and one assessing edoxaban in this therapeutic review. Although two articles did include apixaban and dabigatran, the results in these studies were presented collectively by drug class, therefore individual drug data is limited. Both apixaban and dabigatran would not be deemed favourable in the patient population at KCH owing to the twice-daily dosing, impacting compliance in the post-operative period, where patients are commenced on multiple new drugs.
Recommendations
Although the literature evaluated in this review (n=10) supports the potential use of DOACs in PVT, there is still limited quality data and patient variability remains broad, making universal recommendations difficult. Rivaroxaban is described in the literature as being a possible safe and effective anticoagulant in PVT. However, an individualised and patient-centred approach must be undertaken when assessing the suitability of DOACs in patients with PVT. The degree of liver dysfunction, past medical history and the need for a DOAC must be weighed up meticulously, factoring in patient choice and impact on compliance. Certain groups may not be deemed suitable; for example, Child-Pugh B and C, or patients who have had a liver transplant, whom are at higher risk of bleeding and where data for their use is limited. Routine follow up is essential throughout treatment of PVT and following termination of drug.
Following this therapeutic review, KCH would benefit from conducting a service evaluation to ascertain current local practice of prescribing DOACs in PVT and outcomes in the follow-up period.
Supplementary information

- 1Costache R, Dragomirică A, Dumitraș E, et al. Portal vein thrombosis: A concise review (Review). Exp Ther Med. 2021;22. doi:10.3892/etm.2021.10191
- 2EASL Clinical Practice Guidelines: Vascular diseases of the liver. Journal of Hepatology. 2016;64:179–202. doi:10.1016/j.jhep.2015.07.040
- 3DeLeve LD, Valla D-C, Garcia-Tsao G. Vascular disorders of the liver. Hepatology. 2008;49:1729–64. doi:10.1002/hep.22772
- 4De Gottardi A, Trebicka J, Klinger C, et al. Antithrombotic treatment with direct‐acting oral anticoagulants in patients with splanchnic vein thrombosis and cirrhosis. Liver Int. 2016;37:694–9. doi:10.1111/liv.13285
- 5Czuprynska J, Patel JP, Arya R. Current challenges and future prospects in oral anticoagulant therapy. Br J Haematol. 2017;178:838–51. doi:10.1111/bjh.14714
- 6Bauer KA. Pros and cons of new oral anticoagulants. Hematology. 2013;2013:464–70. doi:10.1182/asheducation-2013.1.464
- 7Lixiana 60mg Film-Coated Tablets – Summary of Product Characteristics. Electronic Medicines Compendium. 2018.https://www.medicines.org.uk/emc/product/6905/smpc (accessed Dec 2021).
- 8Pradaxa 150 mg hard capsules – Summary of Product Characteristics . Electronic Medicines Compendium. 2018.https://www.medicines.org.uk/emc/product/4703/smpc (accessed Dec 2021).
- 9Xarelto 20mg film-coated tablets – Summary of Product Characteristics . Electronic Medicines Compendium. 2018.https://www.medicines.org.uk/emc/product/2793/smpc (accessed Dec 2021).
- 10Eliquis 5 mg film-coated tablets – Summary of Product Characteristics . Electronic Medicines Compendium. 2018.https://www.medicines.org.uk/emc/product/2878/smpc (accessed Dec 2021).
- 11CASP checklists. CASP-UK. https://casp-uk.net/casp-tools-checklists/ (accessed Dec 2021).
- 12Nagaoki Y, Aikata H, Daijyo K, et al. Efficacy and safety of edoxaban for treatment of portal vein thrombosis following danaparoid sodium in patients with liver cirrhosis. Hepatol Res. 2017;48:51–8. doi:10.1111/hepr.12895
- 13Naymagon L, Tremblay D, Zubizarreta N, et al. The efficacy and safety of direct oral anticoagulants in noncirrhotic portal vein thrombosis. Blood Advances. 2020;4:655–66. doi:10.1182/bloodadvances.2019001310
- 14Ai M, Dong W, Tan X, et al. Efficacy and safety study of direct-acting oral anticoagulants for the treatment of chronic portal vein thrombosis in patients with liver cirrhosis. European Journal of Gastroenterology & Hepatology. 2020;32:1395–400. doi:10.1097/meg.0000000000001846
- 15Hanafy AS, Abd-Elsalam S, Dawoud MM. Randomized controlled trial of rivaroxaban versus warfarin in the management of acute non-neoplastic portal vein thrombosis. Vascular Pharmacology. 2019;113:86–91. doi:10.1016/j.vph.2018.05.002
- 16Nery F, Valadares D, Morais S, et al. Efficacy and Safety of Direct-Acting Oral Anticoagulants Use in Acute Portal Vein Thrombosis Unrelated to Cirrhosis. Gastroenterol Res. 2017;10:141–3. doi:10.14740/gr806w
- 17Martinez M, Tandra A, Vuppalanchi R. Treatment of acute portal vein thrombosis by nontraditional anticoagulation. Hepatology 2014;60:425–6. doi:10.1002/hep.26998
- 18Pannach S, Babatz J, Beyer-Westendorf J. Successful treatment of acute portal vein thrombosis with rivaroxaban. Thromb Haemost. 2013;110:626–7. doi:10.1160/th13-05-0407
- 19Lenz K, Dieplinger B, Buder R, et al. Successful Treatment of Partial Portal Vein Thrombosis (PVT) with Low Dose Rivaroxaban. Z Gastroenterol. 2014;52:1175–7. doi:10.1055/s-0034-1385171
- 20Yang H, Kim SR, Song MJ. Recurrent acute portal vein thrombosis in liver cirrhosis treated by rivaroxaban. Clin Mol Hepatol. 2016;22:499–502. doi:10.3350/cmh.2016.0016
- 21Ponziani FR, De Candia E, De Cristofaro R, et al. Portal vein thrombosis occurrence in a cirrhotic patient during treatment with rivaroxaban. Liver Int. 2017;37:1251–1251. doi:10.1111/liv.13406
- 22Idarucizumab. British National Formulary . https://bnf.nice.org.uk/medicinal-forms/idarucizumab.html (accessed Dec 2021).
- 23Andexanet alfa for reversing anticoagulation from apixaban or rivaroxaban (TA697) . National Institute for Health and Care Excellence. https://www.nice.org.uk/guidance/ta697 (accessed Dec 2021).
- 24Zhou J-B, Luo B-Y, Liu C-W, et al. Effects of early antiplatelet therapy after splenectomy with gastro-oesophageal devascularization. ANZ Journal of Surgery. 2018;88:E725–9. doi:10.1111/ans.14395


