VSEVOLOD ZVIRYK / SCIENCE PHOTO LIBRARY
Open access article
The Royal Pharmaceutical Society has made this article free to access in order to help healthcare professionals stay informed about an issue of national importance.
To learn more about coronavirus, please visit: https://www.rpharms.com/coronavirus
After reading this article you should be able to:
- Understand the basic pathophysiology of COVID-19 pneumonia;
- Identify clinical symptoms that may suggest disease progression or require urgent medical attention;
- Describe evidenced-based and supportive therapies for management;
- Appreciate the complexity of managing COVID-19 viral pneumonia and COVID-19 acute respiratory distress syndrome in the acute hospital setting and intensive care unit.
The COVID-19 pandemic has had a significant global impact. At the date of publication, around 203 million people across the world have tested positive for the novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) since it was first identified in December 2019[1].
The most critically unwell patients require urgent admission to an intensive care unit (ICU), often because of severe type 1 respiratory failure with gross hypoxia, meaning that they require mechanical ventilation[2]. Severe respiratory complications, such as COVID-19 pneumonia and/or COVID-19 associated acute respiratory distress syndrome (CARDS), contribute to high ICU mortality rates and affect 15–30% of hospitalised COVID-19 patients[2]. At the peak of the pandemic, the ICU mortality rate for COVID-19 patients reached 58%[2].
Fortunately, as knowledge has expanded and new evidence-based therapies have emerged, associated ICU mortality rates in the UK have fallen to around 37% at 28 days[3]. The number of COVID-19 ICU admissions has also decreased — mostly owing to the successful rollout of the UK COVID-19 vaccination programme[4]. However, the emergence of the more transmissible Delta variant, coupled with the recent easing of lockdown restrictions, has contributed to a third wave of COVID-19 infections in the UK[4,5]. This could potentially lead to an increase in the number of serious complications, such as severe COVID-19 pneumonia and CARDS.
It is therefore important that pharmacists can recognise the signs and symptoms of these diseases, and know how to appropriately manage patients presenting to community pharmacy and primary care.
This article provides an overview of COVID-19 pneumonia and CARDS, summarising the pathophysiology, diagnosis and evidenced-based management strategies.
Effects of COVID-19 on the respiratory system
Human SARS-CoV-2 is a single stranded RNA-enveloped virus, which causes COVID-19 infection. It is thought to have originated in animals owing to the high genetic similarity of the same virus found in bats and pangolins[2,6]. It is primarily a disease of the respiratory tract with entry into the human host via this route[2,7].
SARS-CoV-2 causes damage to the alveolar epithelial lining, impeding oxygen (O2) and carbon dioxide exchange[8]. Damage to the alveoli and a disrupted vascular endothelium, as a consequence of the inflammatory response, causes moderate to severe respiratory failure, requiring ICU admission, intubation and mechanical ventilation[7].
COVID-19 pneumonia and acute respiratory distress syndrome
All patients admitted to ICU are at an increased risk of developing acute respiratory distress syndrome (ARDS). ARDS occurs frequently in the ICU and is characterised by fluid build up in the alveoli, resulting in an insufficient supply of oxygen from the lungs to the vital organs[8]. An ARDS-like pathophysiology has been observed in COVID-19 patients admitted to the ICU — CARDS[6].
The main components of CARDS are destruction of alveolar lining, endothelial damage and a high prevalence of thromboembolic events, including pulmonary embolism[8,9].
The principal difference between CARDS and classic ARDS is the high coagulation burden associated with CARDS[10]. Although several unique pathophysiological features are also postulated in CARDS, such as intravascular thrombosis, endothelial dysfunction and excessive blood flow to collapsed lung tissue[2].
There is also sigificant overlap between CARDS and COVID-19 pneumonia, defined as SARS-CoV-2 infection confirmed by:
- A positive polymerase chain reaction (PCR) test;
- Pulse oximetry showing oxygen saturation (SpO2) of <94% on room air;
- Ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) of <39.9kPa;
- A respiratory rate of >30 breaths/minute, or >50% lung infiltrates observed on radiological imaging[11].
There is not a clear distinction between severe COVID-19 pneumonia and CARDS, which can confound diagnosis[10].
Diagnosis and investigation
SARS-CoV-2 infection is confirmed via a positive COVID-19 PCR test or lateral flow test. PCR tests are generally viewed as the ‘gold standard’ for COVID-19 diagnosis; however, lateral flow tests are cheaper, portable and provide rapid results. Studies to determine their level of sensitivity and specificity are ongoing[12].
Similarly to community acquired pneumonia, COVID-19 pneumonia is diagnosed using a combination of chest X-ray (CXR), blood tests and clinical history[13].
Radiological imaging
While no single feature of COVID-19 infection on the CXR is specific or diagnostic, viral pneumonia caused by SARS-CoV-2 presents as bilateral, peripheral, non-lobar ground glass opacities (hazy, grey areas) as shown in the Figure below.
CARDS is typically diagnosed by chest X-ray and regular CT-scans for in-depth assessment. In the ICU, pulmonary embolism is diagnosed by CT-pulmonary angiogram.
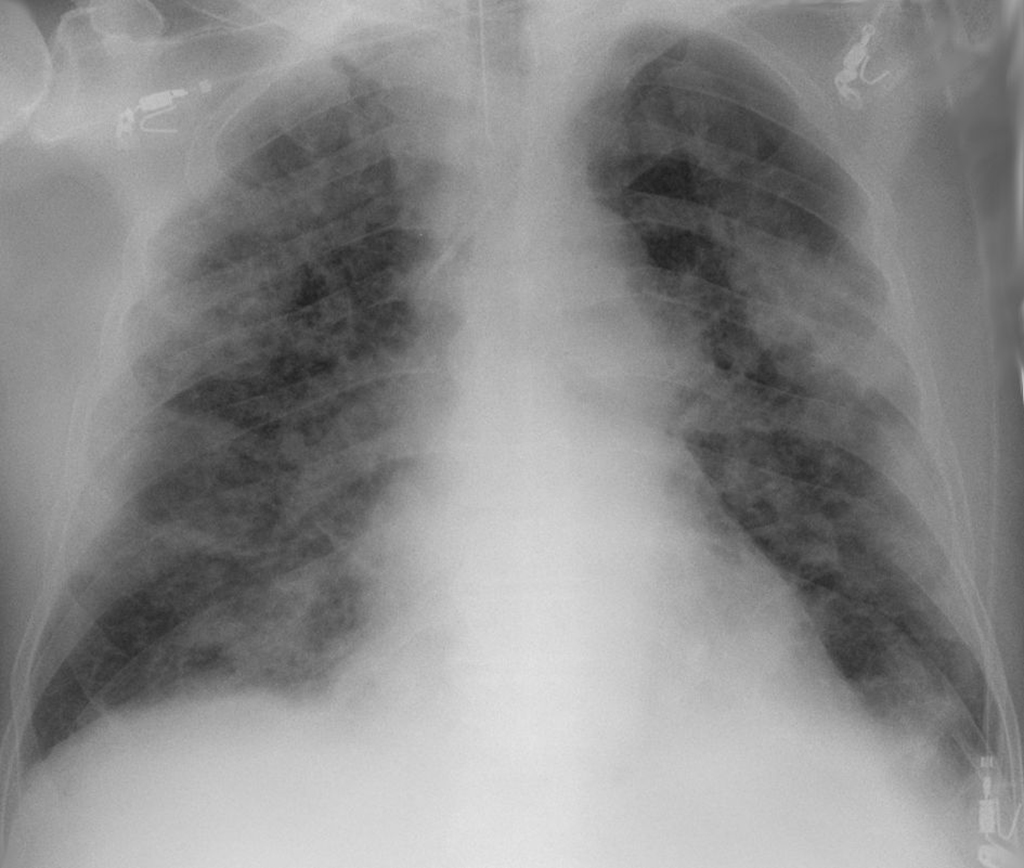
Wikimedia.org
Oxygen saturation
Oxygen saturation (SpO2) is an important diagnostic tool for COVID-19 pneumonia, and measures the amount of haemoglobin-bound oxygen and free oxygen. The partial pressure of arterial O2 (PaO2) is measured by arterial blood gas monitoring. In healthy individuals, SpO2 is usually >95%. In patients with chronic lung disease or sleep apnoea, SpO2 can range between 88–92%[14].
In SARS-CoV-2 infection, patients have low SpO2 levels. A phenomenon known as ‘silent hypoxia’ is reported, where patients present with minimal symptoms yet have significantly reduced pulse oximetry readings below 91%, particularly in the short term[2,15].
Additional investigations
A pattern of characteristic abnormalities — such as a raised C-reactive protein (CRP), a low lymphocyte count and a raised ferritin level — have been identified in patients with severe disease and could stratify patients’ risk[2].
Assessment and referral
Patients with COVID-19 typically present with a dry, persistent cough; temperature >38oC and loss or change in sense of taste and smell (anosmia)[11,13]. A persistent high temperature, loss of appetite, confusion/delirium and reduced urine output are all causes for referral[13]. Patients with COVID-19 pneumonia/CARDs may also feel breathless and complain of tiredness, malaise, headache and a flu-like illness.
In community and primary care, pharmacists should assess the severity of respiratory symptoms and determine the need for referral by asking the patient to describe their chest symptoms alongside the following questions:
- When do you experience breathlessness?
- Are you able to breathe comfortably when resting?
- Do you have difficulty finishing sentences without taking extra breaths?
- Do you avoid speaking because you are too breathless?
- Do you experience any chest tightness?
- How does your breathlessness affect your ability to carry out your usual daily activities?[16]
Pharmacists should refer patients immediately if patients are experiencing the following symptoms:
- Unable to breathe at a comfortable rate on moving or at rest;
- Rapid breathing, i.e. >30 breaths per minute;
- Unable to finish a sentence without taking extra breaths;
- May avoid speaking;
- Feeling ‘tight chested’;
- Unable to read or watch TV because they are too focused on breathing or feel too unwell;
- Coughing up blood;
- Blue lips[12].
Patients with a consistent SpO2 of 93–94% should contact their GP or NHS 111 as soon as possible; those with an SpO2 of 92% or below should attend A&E or call 999 immediately[16]. Pharmacists should be aware of silent hypoxia, and variations in the measurement of SpO2 and overestimation in black African/Caribbean individuals[2,17]. For more information on how to assess and manage patients in community or primary care, see ‘Community management of pneumonia and suspected COVID-19’.
Pharmacists should also be aware that selected patients are more likely to develop severe COVID-19 pneumonia, including frail people, older people, those with multiple comorbidities (including diabetes and cardiovascular disease), impaired immunity, male gender, obesity or a reduced ability to cough and clear secretions[12].
Neither the symptoms described or the list of patients at risk of severe disease are exhaustive, and pharmacy professionals should refer to the NICE COVID-19 rapid guidelines[11].
Management
Treatment of COVID-19 pneumonia and CARDs occurs mainly in secondary care, although milder cases of COVID-19 pneumonia have been managed in the community. Evidence around COVID-19 therapies is rapidly being updated; therefore, pharmacists must possess in-depth knowledge of treatment strategies[18].
Up until June 2020, the mainstay of treatment for COVID-19 pneumonia and CARDS was symptomatic, supportive therapy, including oxygen and mechanical ventilation. More recently, evidence generated from the Randomised Evaluation of COVID-19 Therapy (RECOVERY) and Randomised, Embedded, Multi-factorial, Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) landmark trials are being used to guide therapy[19,20]. The adaptive (multi-domain) design of these studies allows interim results from domains to be shared as soon as they provide evidence of sufficient certainty to affect international treatment strategies[19,20].
National guidance on the management of COVID-19 has also been published by the National Institute for Health and Care Excellence (NICE) and NHS England[13]. Although the NHS was overwhelmed with COVID-19 cases, patient enrolment in these vital studies continued as multidisciplinary teams recognised that evidence for novel therapy was essential.
Additional information about COVID-19 therapy trials can be found here.
Pharmacological therapies
The majority of pharmacological therapies, which have — to date — proven to be effective in the management of COVID-19, target the hyper-inflammatory processes that occur in moderate to severe COVID-19 disease[16,17]. Evidence shows that the following therapies can be given concomitantly, provided each is safe and appropriate for the patient at the time of presentation[11].
Dexamethasone
Interim results from the RECOVERY trial support treatment with dexamethasone, a well-known, readily available and affordable corticosteroid[17].
Dexamethasone has been shown to reduce mortality in ventilated patients by one-third, and by one-fifth in hospitalised patients requiring oxygen therapy[17]. The absolute risk reduction in patients receiving dexamethasone was 3%, with a number needed to treat of 33 to save one life. However, no benefit was reported in patients who did not require oxygen treatment[19].
The most effective dose of dexamethasone for the treatment of COVID-19 pneumonia remains under investigation — at present, patients are prescribed 6mg once daily for 10 days in line with the available evidence; however, there are studies investigating whether higher doses of dexamethasone (12mg once daily) are more effective[21].
Prolonged steroid treatment causes immunosuppression, predisposing patients to risk of bacterial and fungal infections with resistant organisms. Pharmacists are central to upholding effective antimicrobial stewardship by reviewing the appropriateness of antimicrobials prescribed, along with patient education on administration and adherence.
Steroid-induced hyperglycaemia can also occur and, in many cases, insulin therapy is required. Evidence shows that glucose control (aiming for blood glucose levels between 6–10mmol/L) for patients in intensive care leads to better outcomes. Pharmacist monitoring of blood glucose should occur throughout dexamethasone therapy[22].
Remdesivir
Treatment with remdesivir, an adenosine nucleotide prodrug — previously developed for the treatment of the Ebola virus — should be considered for adults with COVID-19 pneumonia, who are in hospital and receiving supplemental oxygen but who are not mechanically ventilated[23].
A five-day course of intravenous remdesivir (200mg on day 1, then 100mg daily) reduces time to recovery by one-third but no mortality benefit has been reported[24]. Caution should be exercised in patients with severe liver or renal impairment[23].
Tocilizumab
Interleukin-6 (IL-6) is an inflammatory cytokine that plays a key inflammatory role in COVID-19 pneumonia/CARDS[23]. The RECOVERY and REMAP-CAP trials found that tocilizumab, an IL-6 inhibitor, licensed in the treatment of rheumatoid arthritis, reduces mortality, time to ICU and hospital discharge and, in patients receiving oxygen, reduces the chance of progressing to mechanical ventilation[25].
An 8mg/kg dose of tocilizumab by intravenous infusion is recommended for patients who require supplemental oxygen, have a CRP ≥75mg/L and are within 48 hours of advanced respiratory support[25]. Caution is advised in patients who have active bacterial or viral infection (other than SARS-COV-2) as these can be exacerbated by tocilizumab-induced immunosuppression[26]. Tocilizumab is reported to suppress CRP production for up to three months; therefore, caution is advised in using CRP to exclude sepsis in tocilizumab-treated patients[27].
Therapies are assessed continually in COVID-19; therefore, a clear handover and medicines reconciliation upon transfer between care settings is essential[28]. A recently reported intervention is Regeneron, combination monoclonal antibody treatment, from the RECOVERY study, which provided clinical benefit in patients who had failed to mount an adequate immune response[29].
Additional therapies
Patients admitted to ICU with COVID-19 pneumonia and CARDS require deep sedation and paralysis for prolonged periods[30]. There is a high risk of iatrogenic withdrawal upon sedation weaning and delirium. Pharmacists should collaborate with the multidisciplinary team to devise sedation weaning plans to reduce these risks[31].
Oxygen
Oxygen is essential in the treatment of severe COVID-19 pneumonia/CARDs and is commonly started when SpO2 is <94% on room air via nasal cannula[2].
Advanced respiratory support, including high flow nasal oxygen [HFNO] or continuous positive airways pressure (CPAP), is considered when an SpO2 ≥94% on 40% oxygen is unachievable[11]. If maximal non-invasive respiratory support fails to achieve adequate SpO2, then intubation and ventilation may be necessary[11].
The Recovery-RS (Respiratory Support) trial is comparing the effectiveness of three ventilation methods in patients with COVID-19 (CPAP versus high flow nasal oxygen (HFNO) versus standard care)[32]. The results from RECOVERY-RS are important in identifying optimum ventilation methods.
Thromboprophylaxis and anticoagulation
COVID-19 induces a hypercoagulable state, especially in severe disease, and it is essential that appropriate thromboprophylaxis and anticoagulation strategies are adhered to in hospitalised patients[2].
Full dose anticoagulation is recommended in adults with COVID-19 infection receiving supplemental oxygen, but not in those receiving advanced respiratory support (HFNO, CPAP, non-invasive or mechanical ventilation).
In the ICU, the REMAP-CAP trial reported that treatment dose anticoagulation is futile, and is associated with a higher rate of bleeding events in critically ill patients[28]. Thromboprophylaxis with either standard or intermediate dose low molecular weight heparin (i.e standard prophylactic dose for those acutely unwell administered twice daily instead of once daily) is recommended. Trials are ongoing to establish the optimum dose of thromboprophylaxis in the ICU cohort[33].
Follow up and monitoring
Persisting and long-lasting symptoms are reported after SARS-CoV-2 infection. The overarching term most commonly used is ‘long COVID’[34]. The challenge after severe COVID-19 pnemonia and CARDs is that ICU admission can be prolonged, and symptoms of ‘long COVID’ are similar or overlap with post intensive care syndrome, which includes profound weakness and cognitive decline. This means a large number of patients are likely to require long-term rehabilitation[35].
During extended admissions, ongoing medicines reconciliation and communication between care settings is vital. It is particularly important that details regarding inpatient corticosteroid and IL-6 inhibitor therapy is communicated to the patient’s care providers following discharge from ICU, to step down wards and primary care. The box summarises how pharmacists can facilitate this[28].
Box: How pharmacists can support COVID-19 pneumonia and CARDs patients on discharge
Best practice recommendations for effective information sharing and integrated working:
- Ensure accuracy in prescribing between transfer of care. Caution should be taken when discontinuing unnecessary medications (e.g antipsychotics) on step down from ICU. Reconciliation of long term therapy is necessary to mitigate risks of unintentional discontinuation;
- Include details of prescribed therapy (e.g. dexamethasone and tocilizumab), as well as baseline and follow-up test results in clinical records/rehabilitation plans. These should be shared promptly between services during multidisciplinary meetings;
- Provide patients with a copy of their care plans or records to keep, including discharge letters, clinical records, rehabilitation plans and prescriptions;
- Advocate for one healthcare professional or team to provide care for the same patient as much as possible to ensure continuity of care;
- Provide medication counselling, and signpost patients to appropriate health and social care services after discharge from hospital.
Case study
The following is a case study to illustrate the typical presentation and ICU admission of a patient diagnosed with COVID-19 pneumonia. The case is fictional but has been based on several of the COVID-19 patients admitted to the ICU at King’s College Hospital.
Mrs AB is a female aged 44 years with a history of type 2 diabetes, hypertension and depression. Prior to experiencing COVID-19 pneumonia, she was fit, well and working full-time. Mrs AB felt unwell five days before hospital presentation, with aches and pains, intermittent fevers, headache and cough. She also reported loss of taste and smell. On presentation to the emergency department, her initial observations were:
| SPO2: 83% | White cell count: 5.10 x 106 |
| Temperature: 38.7oC | Platelets: 302 |
| C-reactive protein: 210mg/L | Lymphocytes: 0.95 |
| Creatinine 52mmol/L | International Normalised Ratio: 1.00 |
A CXR reported bilateral infiltrates — characteristic of COVID-19 pneumonia — that was confirmed by a positive SARS-CoV-2 PCR test.
Mrs AB was admitted to a medical ward and received treatment with HFNO. She was prescribed dexamethasone 6mg daily for ten days and a five-day course of remdesivir (200mg on day 1, then 100mg daily thereafter)[24].
Around 24 hours after admission, her CRP was rising and oxygen requirements increasing. A CXR reported deterioration. Mrs AB was transferred to the ICU for intubation and ventilation, with a high clinical suspicion of a pulmonary embolism. Her oxygen requirements at intubation were 100%.
On ICU arrival, a CT pulmonary angiogram scan reported no evidence of pulmonary embolism. Mrs AB was prescribed an intermediate dose thromboprophylaxis for COVID-19. Her treatment with remdesivir was ceased on suspicion that a recent derangement in liver function was secondary to remdesivir.
Mrs AB remained in ICU for 69 days before being discharged to the ward.
Patient history from ICU:
- Rising inflammatory markers, CRP peaked at 467mg/L ferritin peak of 890ng/mL (normal level <200ng/mL in women);
- Challenging oxygenation, ongoing hypoxia — management included deep sedation with fentanyl, propofol and midazolam, paralysis and repositioning in the prone position;
- Secondary effects were low creatinine (owing to loss of muscle mass) and profound myopathy;
- Insulin infusion initiated to manage dexamethasone-induced hyperglycaemia, titrated as per local protocol (blood glucose peak = 20mmol/L).
- Tracheotomy on day 20;
- Development of multi-resistant gram-negative bacteraemia (Klebsiella spp.) and a positive Beta-D glucan result. Treated with meropenem, amikacin and anidulafungin;
- Upon weaning sedation, patient became extremely tachycardic and respiratory rate increased to 50–55 breaths per minute — secondary to iatrogenic withdrawal owing to rapid weaning of high doses of sedation;
- Gradual sedation weaning plan was devised, weaning midazolam and fentanyl infusions over 10–14 days, bridging on to intermittent diazepam and morphine.
Summary
In summary, COVID-19 pneumonia and CARDs are complex, multi-system disorders with a high risk of death. Patients that survive can have significant and lasting consequences. These are outlined in the earlier learning article ‘Managing the long-term effects of COVID-19’. The clinical evidence in COVID-19 pneumonia and CARDS is continually updated. The authors of this article have done their utmost to provide an overview of the most up to date evidence, but acknowledge that the most recent evidence may not have been included at the time of publication.
- 1COVID-19 map. John Hopkins University of Medicine. 2021.https://coronavirus.jhu.edu/map.html (accessed Aug 2021).
- 2Attaway AH, Scheraga RG, Bhimraj A, et al. Severe covid-19 pneumonia: pathogenesis and clinical management. BMJ 2021;:n436. doi:10.1136/bmj.n436
- 3Ireland N. ICNARC report on COVID-19 in critical care. ICNARC. 2021.https://www.icnarc.org/DataServices/Attachments/Download/609422cd-d851-eb11-912d-00505601089b (accessed Aug 2021).
- 4Callaway E. Delta coronavirus variant: scientists brace for impact. Nature 2021;595:17–8. doi:10.1038/d41586-021-01696-3
- 5Ventura D, Aith F, Reis R. The catastrophic Brazilian response to COVID-19 may amount to a crime against humanity. BMJ. 2021.https://blogs.bmj.com/bmj/2021/04/05/the-catastrophic-brazilian-response-to-covid-19-may-amount-to-a-crime-against-humanity/ (accessed Aug 2021).
- 6Marini JJ, Gattinoni L. Management of COVID-19 Respiratory Distress. JAMA 2020;323:2329. doi:10.1001/jama.2020.6825
- 7Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med 2020;46:1099–1102. doi:10.1007/s00134-020-06033-2
- 8Domenighetti G, Stricker H. [Respiratory distress syndrome and hypothermia in Wernicke’s encephalopathy]. Schweiz Med Wochenschr 1985;115:1764–6.https://www.ncbi.nlm.nih.gov/pubmed/4089574
- 9Sakr Y, Giovini M, Leone M, et al. Pulmonary embolism in patients with coronavirus disease-2019 (COVID-19) pneumonia: a narrative review. Ann Intensive Care 2020;10. doi:10.1186/s13613-020-00741-0
- 10Grasselli G, Tonetti T, Protti A, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. The Lancet Respiratory Medicine 2020;8:1201–8. doi:10.1016/s2213-2600(20)30370-2
- 11COVID-19 treatment guidelines. National Institutes of Health. 2021.https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed Sep 2021).
- 12Mahase E. Covid-19: Innova lateral flow test is not fit for “test and release” strategy, say experts. BMJ 2020;:m4469. doi:10.1136/bmj.m4469
- 13NICE guideline NG191. COVID-19 rapid guideline: managiong COVID-19. National Institute for Health and Care Excellence. 2021.https://www.nice.org.uk/guidance/ng191 (accessed Aug 2021).
- 14McNicholas W, Kent, Mitchell. Hypoxemia in patients with COPD: cause, effects, and disease progression. COPD 2011;:199. doi:10.2147/copd.s10611
- 15Chandra A, Chakraborty U, Pal J, et al. Silent hypoxia: a frequently overlooked clinical entity in patients with COVID-19. BMJ Case Rep 2020;13:e237207. doi:10.1136/bcr-2020-237207
- 16Symptoms of coronavirus (COVID-19). NHS. 2021.https://www.nhs.uk/conditions/coronavirus-covid-19/symptoms/ (accessed Aug 2021).
- 17Sjoding MW, Dickson RP, Iwashyna TJ, et al. Racial Bias in Pulse Oximetry Measurement. N Engl J Med 2020;383:2477–8. doi:10.1056/nejmc2029240
- 18Shulman R, McKenzie CA, Landa J, et al. Pharmacist’s review and outcomes: Treatment-enhancing contributions tallied, evaluated, and documented (PROTECTED-UK). Journal of Critical Care 2015;30:808–13. doi:10.1016/j.jcrc.2015.04.008
- 19Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 2021;384:693–704. doi:10.1056/nejmoa2021436
- 20Angus DC, Derde L, et al. Effect of Hydrocortisone on Mortality and Organ Support in Patients With Severe COVID-19. JAMA 2020;324:1317. doi:10.1001/jama.2020.17022
- 21Mishra GP, Mulani J. Corticosteroids for COVID-19: the search for an optimum duration of therapy. The Lancet Respiratory Medicine 2021;9:e8. doi:10.1016/s2213-2600(20)30530-0
- 22Van den Berghe G, Wouters P, Weekers F, et al. Intensive Insulin Therapy in Critically Ill Patients. N Engl J Med 2001;345:1359–67. doi:10.1056/nejmoa011300
- 23Remdesivir. BNF. 2021.https://bnf.nice.org.uk/drug/remdesivir.html (accessed Aug 2021).
- 24Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 — Final Report. N Engl J Med 2020;383:1813–26. doi:10.1056/nejmoa2007764
- 25Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med 2021;384:1491–502. doi:10.1056/nejmoa2100433
- 26Angriman F, Ferreyro BL, Burry L, et al. Interleukin-6 receptor blockade in patients with COVID-19: placing clinical trials into context. The Lancet Respiratory Medicine 2021;9:655–64. doi:10.1016/s2213-2600(21)00139-9
- 27Mariette X, Hermine O, Tharaux P-L, et al. Effectiveness of Tocilizumab in Patients Hospitalized With COVID-19. JAMA Intern Med Published Online First: 24 May 2021. doi:10.1001/jamainternmed.2021.2209
- 28Barrett NA, Jones A, Whiteley C, et al. Management of long-term hypothyroidism: a potential marker of quality of medicines reconciliation in the intensive care unit†. International Journal of Pharmacy Practice 2012;20:303–6. doi:10.1111/j.2042-7174.2012.00205.x
- 29Horby PW, Mafham M, et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. 2021. doi:10.1101/2021.06.15.21258542
- 30Page V. Sedation in mechanically ventilated patients with COVID-19. The Lancet Respiratory Medicine 2021;9:218–9. doi:10.1016/s2213-2600(20)30570-1
- 31Arroyo-Novoa CM, Figueroa-Ramos MI, Puntillo KA. Opioid and Benzodiazepine Iatrogenic Withdrawal Syndrome in Patients in the Intensive Care Unit. AACN Advanced Critical Care 2019;30:353–64. doi:10.4037/aacnacc2019267
- 32Perkins GD, Couper K, Connolly B, et al. RECOVERY- Respiratory Support: Respiratory Strategies for patients with suspected or proven COVID-19 respiratory failure; Continuous Positive Airway Pressure, High-flow Nasal Oxygen, and standard care: A structured summary of a study protocol for a randomised controlled trial. Trials 2020;21. doi:10.1186/s13063-020-04617-3
- 33Chandra A, Chakraborty U, Ghosh S, et al. Anticoagulation in COVID-19: current concepts and controversies. Postgrad Med J 2021;:postgradmedj-2021-139923. doi:10.1136/postgradmedj-2021-139923
- 34Managing the long-term effects of COVID-19. Pharmaceutical Journal Published Online First: 2021. doi:10.1211/pj.2021.1.86280
- 35Wade DT. Rehabilitation after COVID-19: an evidence-based approach. Clin Med 2020;20:359–65. doi:10.7861/clinmed.2020-0353