Shutterstock.com
After reading this article, you should be able to:
- Understand the mechanism of action and indications for digoxin;
- Understand common interactions with the use of digoxin;
- Recognise when monitoring of digoxin is indicated;
- Understand the management of digoxin toxicity.
Digoxin is a chemical derived from the plant Digitalis purpurea, more commonly known as foxglove, and belongs to the class of medicines known as cardiac glycosides[1]. Digoxin is used to treat a variety of cardiovascular conditions and provides inotropic activity (i.e. alters the force or energy of the heart muscle’s contractions) in the management of heart failure, and negative chronotropic effect (i.e. slows the heart rate) in treating abnormal heart rhythms, such as atrial flutter and atrial fibrillation[2].
Around 3 million prescriptions are generated for digoxin in England each year and around 1% of patients will develop toxicity — a risk that increases with age[3,4]. The use of digoxin in the treatment of heart failure has declined in recent years owing to findings from observational studies showing safety concerns linked with increased mortality rates with its use[2,5,6]. However, the DIG trial — the largest randomised controlled trial of digoxin in patients with heart failure (n= 3,397) — showed digoxin did not reduce overall mortality, but reduced the rate of hospitalisation both overall and for worsening heart failure. These findings more precisely define the role of digoxin in the management of chronic heart failure[2].
Digoxin toxicity is often accidental and occurs because of inappropriate dosing, lack of appropriate serum digoxin monitoring, and lack of recognition of signs/symptoms of digoxin toxicity by both the patient and healthcare professionals[7]. Pharmacists therefore need to be aware of digoxin use and signs/symptoms of toxicity when treating patients with cardiovascular conditions.
Mechanism of action
The main action of digoxin is the inhibition of the sodium–potassium (Na+ K+) pump by inhibiting the Na+ K+-ATPase enzyme present in the membrane of cardiac cells (see Figure)[1–3]. Owing to the interplay between these electrolytes, there may be a considerable increase in potency when the extracellular potassium concentration is low, as opposed to hyperkalaemia having the opposite effect[1–3].
Digoxin has a direct action on conduction through increased vagal tone, resulting in reduced sympathetic tone and diminished impulse conduction rate through the atria and atrioventricular (AV) node; therefore, the major effect of digoxin is reduction of ventricular rate[1–3].
Digoxin exhibits its toxic effects by poisoning the Na/K ATPase pathway that results in an increase in intracellular sodium, leading to increased intracellular calcium through decreasing calcium expulsion within the sodium–calcium cation exchanger[7,8].
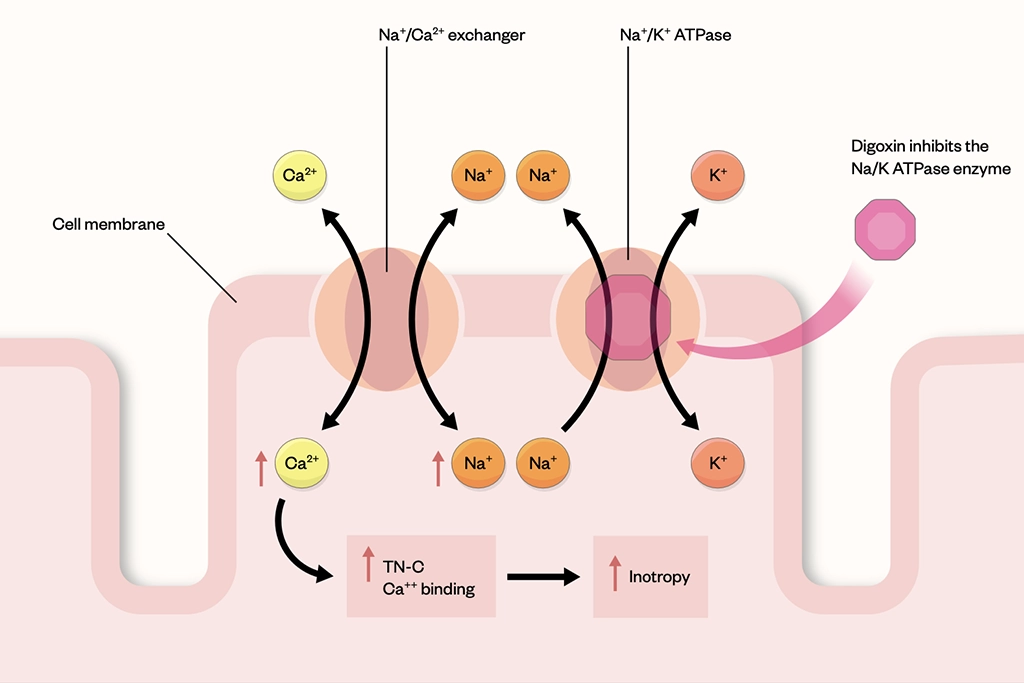
Inhibiting the Na/K ATPase enzyme results in a reduction of intracellular potassium, increase in intracellular sodium and a corresponding augmented calcium ion influx and thus an increase in the availability of calcium at the time of excitation-contraction coupling
Initiation
Patients with atrial fibrillation and flutter are usually given higher doses of digoxin to rapidly control heart rhythm followed by lower maintenance doses (see Table 1)[3,4]. The primary effect of digoxin is to slow down AV conduction, leading to a reduction in ventricular response. Patients with heart failure who are maximised on prognostic therapies (e.g. beta blockers, angiotensin-converting enzyme inhibitors) and remain symptomatic with reduced ejection fraction may have digoxin added. In heart failure, digoxin slows AV conduction to allow for increased ventricular filling time and to strengthen the force of ventricular contraction.
Administration of intravenous digoxin produces pharmacological effects within 5–30 minutes, while oral administration produces an onset of effect within 30 minutes to 2 hours[1–3].
Dose reduction is managed based on patients’ renal function, interacting medicines either started or stopped, digoxin serum levels if available or lack of response. Lower doses are then restarted and titrated based on therapeutic action.
Monitoring
Digoxin has a narrow therapeutic window, meaning there is a small margin between the benefit of its effects and toxicity; therefore, monitoring is an important part of its use. Testing serum digoxin levels can indicate whether patients are compliant, as very low levels of serum digoxin could indicate that patients are not taking digoxin as prescribed.
Serum digoxin levels should be measured in newly initiated patients after seven days, as this is normally when steady state is reached. Monitoring of serum digoxin is usually carried out after any dose changes, suspected signs and symptoms of toxicity, or changes in therapy (i.e. concomitant therapy, such as the antiarrhythmic drugs amiodarone and verapamil, which can interact with digoxin therapy and affect plasma concentration of digoxin)[9]. Routine monitoring is not usually recommended in patients who are therapeutically stable and there is no evidence from randomised controlled trials to suggest routine monitoring provides better outcomes for patients[10].
Monitoring is usually indicated in patients with poor renal function, and very unwell patients (e.g. those with an acute infection, who are older or have comorbidities) with electrolyte disturbances or suspected overdose[9].
If toxicity is suspected, serum digoxin radioimmunoassay can be performed to assess digoxin levels in the blood. Blood should be taken 6 hours or more after the last dose of digoxin was administered, but ideally 8–12 hours afterwards[10]. This is usually how long it takes to achieve equilibrium of serum concentration with the myocardium and plasma to exert digoxin’s effects[7]. Most data suggest that typical digoxin serum ranges from 0.7 nanograms/mL to 2.0nanograms/mL depending on the indication as this balances the beneficial effects of the drug with lower risk of toxic symptoms[10]. Above this range there is an increased risk of developing signs and symptoms likely of digoxin toxicity[10].
Side effects
Patients reporting side effects should be carefully assessed and referred for further investigations when required. This would involve taking a thorough clinical history and, where possible, a blood test to check urea, electrolytes and serum digoxin levels. Patients may present to local community pharmacies with symptoms (see below); when digoxin toxicity is suspected, patients should be referred to A&E. This is especially important in older people, owing to the likelihood of additional comorbidities, such as chronic obstructive pulmonary disease, increasing the risk of toxicity and making management more difficult[8–10].
The following symptoms could suggest toxicity and patients should be promptly referred for further assessment:
- Visual disturbances (e.g. blurred vision, changes in how colours look, seeing spots);
- Confusion;
- Nausea, vomiting and diarrhoea;
- Tachycardia;
- Fatigue and weakness[3,4].
The summary of product characteristics should be consulted for a full list of side effects reported with the administration of digoxin[8].
General features reported with digoxin toxicity (e.g. nausea, vomiting, diarrhoea, and general malaise) can develop within 1–2 hours of acute overdose[7,11]. The cardiac adverse effects may take more than 12 hours to fully develop[8,11].
Onset of toxicity from chronic therapy can be variable but is often preceded by dehydration from other causes/drug interactions[7,11]. Serum digoxin concentrations less than 1.5 nanograms/mL in the absence of hypokalaemia usually indicate that digoxin toxicity is very unlikely[7]. Serum digoxin concentrations greater than 3 nanograms/mL is suggestive of toxicity[7,11].
Contraindications
In the management of rate control in atrial fibrillation, there are several agents that should be used first line, prior to digoxin, depending on individual patient clinical needs. These agents commonly include beta blockers and calcium channel blockers. Digoxin is added only if adequate rate control is not achieved[12].
As digoxin can affect heart rate and force of contraction, it is contraindicated in patients with abnormal rhythms, such as intermittent complete heart block or second-degree AV block, and supraventricular arrythmias (e.g. Wolff-Parkinson-White syndrome)[8].
Digoxin is contraindicated for use in patients with known hypertrophic obstructive cardiomyopathy owing to its effect on the force of contraction and heart rate. Hypersensitivity to the active substance, other digitalis glycosides or any excipients used to formulate the drug are also contraindications for its use[8].
Interactions
There are several food and drug interactions that can lead to changes in digoxin serum concentrations. Patients taking medicines that interact with digoxin should have doses adjusted according to response and levels monitored to ensure appropriate dose adjustments are made.
Some common medicines that interfere with digoxin are:
- Aminoglycosides (e.g. amikacin, gentamicin), which can increase the serum digoxin level owing to their effect on renal function;
- Antiarrhythmics (e.g. amiodarone and verapamil). Amiodarone can double the serum concentration of digoxin, and synergistic effects on heart rate and AV node conduction can occur. Verapamil can increase serum digoxin levels. It is recommended to reduce the dose of digoxin by 30–50% and closely monitor serum digoxin levels to determine effects;
- Macrolides (e.g. azithromycin, clarithromycin and erythromycin) have been reported to increase serum digoxin levels. Patients should be monitored for signs/symptoms of digoxin toxicity;
- Rifampicin reduces digoxin levels by up to 80%. Patients should be monitored closely for under digitalisation and serum digoxin levels should be monitored with dose adjustments as appropriate[8,11,13].
A detailed list of all the interactions and their mechanisms can be found on MedicinesComplete[11].
Large quantities of fibre have been shown to reduce digoxin bioavailability by around 20%. Patients should be advised to eat small amounts of fibre-rich meals while taking digoxin or to consider alternate timing when taking the medicine[8,11].
Management of digoxin toxicity
Toxicity can be more severe in patients who are taking cardiotoxic substances (e.g. beta blockers and rate-limiting calcium channel blockers)[7,13]. The extent of severity can be made worse owing to poor renal function and increasing age. Prompt treatment of digoxin toxicity is vital; death can occur because of complications such as ventricular arrhythmias, conduction impairment or heart failure[7,11].
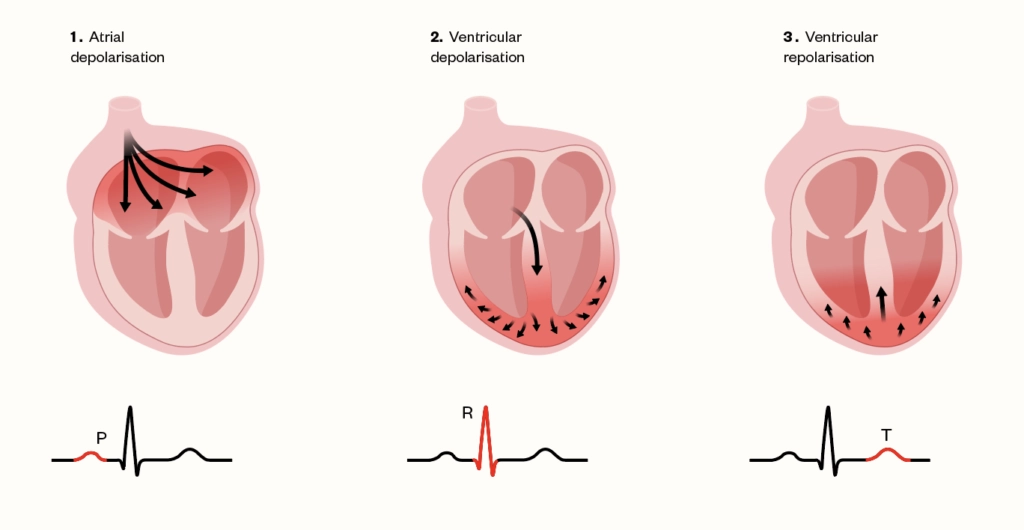
All patients presenting with suspected digoxin toxicity should have a 12-lead echocardiogram (ECG) performed. Overdose usually causes a marked bradycardia with ECG changes, such as PR and QRS prolongation (representing atrial and ventricular depolarisation respectively), see Figure 2[14]. Sinus arrest, varying degrees of AV with dissociation or escape rhythms may occur, including paroxysmal atrial tachycardia with AV block, junctional tachycardia, frequent ventricular ectopics and bigeminy (alternation between a regular heartbeat and an extra, skipped heartbeat)[8,11,15]. In serious cases of toxicity, ventricular tachycardia and ventricular fibrillation may occur. Adverse arrythmias are most common in patients if there is pre-existing heart disease or in electrolyte disturbances, such as hypokalaemia[8,11,15].
Discussion with intensivists is required for patients who are symptomatic and/or have an abnormal ECG. Rapid fluid resuscitation may be required in patients with hypovolaemia (i.e. decreased blood volume), being cautious in those with a history of left ventricular dysfunction. Hypovolaemia caused by dehydration can be a result of persistent nausea, vomiting and diarrhoea, and a result of excessive diuresis in patients with heart failure[10]. Where appropriate, fluid resuscitation will aid in reducing plasma digoxin concentrations. A blood sample should be obtained from the patient to assess digoxin concentration in view of suspected toxicity[10].
The information in the Box can support pharmacists to make informed decisions when managing patients taking digoxin and assessing whether toxicity is suspected.
Box: Warning signs of digoxin toxicity
- Presence of any of the previously outlined symptoms (visual disturbance, e.g. green/yellow tinge, nausea/vomiting-associated, confusion/disorientation);
- Heart rate: is the heart rate <60 beats per minute? Measure to assess and make a clinical decision to refer for further medical input;
- Concomitant medicines taken by the patient that can alter the heart rate or serum potassium levels (e.g. amikacin, gentamicin, amiodarone and verapamil);
- Assess and check serum potassium levels where possible. Has the patient had a recent blood test? What serum potassium level was reported?[8]
Management of digoxin toxicity usually involves correction of electrolytes, such as in hyperkalaemia. Severe cases will require insulin and dextrose for the management of hyperkalaemia. Local policies may vary, but an example would be to administer 10 units of short acting insulin with 100mL of 20% dextrose intravenously over 5 minutes. If hyperkalaemia is not improving, these doses can be repeated as clinically indicated[8]. To stabilise the cardiac membrane excitability, slow, intravenous injection of 10mL of 10% calcium chloride (6.8mmol) with continuous cardiac monitoring is required. Alternatively, 10mL of 10% calcium gluconate (2.3mmol) can be administered by slow intravenous injection. The dose can be repeated in 5–10 minutes if there are no improvements in the ECG up to a maximum of 30mL (6.8mmol)[8].
If the patient is found to have toxic levels of digoxin (>3 nanograms/mL), digoxin-specific antibody FAB fragments (DigiFab; Veriton Pharma Limited) can be administered as an intravenous bolus dose (see Table 2)[16]. DigiFab can also be used when patients present with poisoning from digoxin associated with severe bradyarrythmia or severe hyperkalaemia (e.g. serum potassium levels greater than 6.5mmol/L), which is resistant to adequate rehydration and conventional treatments[16]. DigiFab rapidly reduces serum-free digoxin to undetectable levels within 30 minutes[17]. Serum samples for digoxin concentration should be obtained before DigiFab administration. The above are administered in view of digoxin causing a shift of potassium from the inside to the outside of the cell[16]. When the toxic effects of digoxin are reversed by DigiFab, potassium shifts back into the cell, resulting in a decline in serum potassium concentration. Therefore, serum potassium concentration should be monitored closely, especially during the first few hours after DigiFab administration[16].
Each vial should be reconstituted with 4mL of sterile water by gentle mixing prior to administration[16].
The incidence of digoxin toxicity can be reduced through appropriate patient education and counselling. Pharmacists should ensure that patients understand and are counselled on the following:
- The correct dose and regimen of digoxin that should be taken — this can be achieved by providing patients with instruction sheets or including digoxin as part of dosette boxes to prevent overdosage/dose omissions. Community pharmacists can assess this through active and regular medicines use reviews;
- Recognising the potential side effects;
- The signs of toxicity and what action should be taken in the event of suspected toxicity. Patients may report their suspected toxicity to their local community pharmacy or GP for further information/review and, in severe cases, should be educated to attend their nearest A&E department.
- 1Digoxin. British Heart Foundation. 2018.https://www.bhf.org.uk/informationsupport/heart-matters-magazine/medical/drug-cabinet/digoxin (accessed Jan 2023).
- 2Ziff OJ, Lane DA, Samra M, et al. Safety and efficacy of digoxin: systematic review and meta-analysis of observational and controlled trial data. BMJ. 2015;:h4451. doi:10.1136/bmj.h4451
- 3The Effect of Digoxin on Mortality and Morbidity in Patients with Heart Failure. N Engl J Med. 1997;336:525–33. doi:10.1056/nejm199702203360801
- 4Digoxin (0201010F0). OpenPrescribing. 2022.https://openprescribing.net/chemical/0201010F0/ (accessed Jan 2023).
- 5Vamos M, Erath JW, Hohnloser SH. Digoxin-associated mortality: a systematic review and meta-analysis of the literature. Eur Heart J. 2015;36:1831–8. doi:10.1093/eurheartj/ehv143
- 6Bavishi C, Khan AR, Ather S. Digoxin in patients with atrial fibrillation and heart failure: A meta-analysis. International Journal of Cardiology. 2015;188:99–101. doi:10.1016/j.ijcard.2015.04.031
- 7Cummings E, Swoboda H. Digoxin Toxicity. National Library of Medicine. 2022.https://www.ncbi.nlm.nih.gov/books/NBK470568/ (accessed Jan 2023).
- 8Digoxin tablets BP 250 micrograms. Electronic medicines compendium. 2020.https://www.medicines.org.uk/emc/product/5773/smpc#CONTRAINDICATIONS (accessed Jan 2023).
- 9Atrial fibrillation: Digoxin. National Institute for Health and Care Excellence. 2022.https://cks.nice.org.uk/topics/atrial-fibrillation/prescribing-information/digoxin/ (accessed Jan 2023).
- 10Digoxin. National Institute for Health and Care Excellence. 2022.https://bnf.nice.org.uk/drugs/digoxin/#indicationsAndDoses (accessed Jan 2023).
- 11Digoxin interactions. MedicinesComplete. 2022.https://about.medicinescomplete.com/#/interactions/stockley?terms=Digoxin (accessed Jan 2023).
- 12Rate Control Medication for Atrial Fibrillation. StopAfib.org. 2022.https://www.stopafib.org/managing-afib/medication-for-afib/control-afib/rate-control-medication/ (accessed Jan 2023).
- 13Currie GM. Pharmacokinetic Considerations for Digoxin in Older People. TOCMJ. 2011;5:130–5. doi:10.2174/1874192401105010130
- 14MEJHOUDI S, JENKAL RLW, Saddik A, et al. Hardware Architecture for Adaptive Dual Threshold Filter and Discrete Wavelet Transform based ECG Signal Denoising. IJACSA. 2021;12. doi:10.14569/ijacsa.2021.0121106
- 15Digoxin. TOXBASE. 2022.https://www.toxbase.org/?ReturnUrl=%2fPoisons-Index-A-Z%2fD-Products%2fDigoxin—————-%2f (accessed Jan 2023).
- 16DigiFab 40 mg/vial, Powder for solution for infusion. Electronic medicines compendium. 2022.https://www.medicines.org.uk/emc/product/13957/smpc (accessed Jan 2023).
- 17Frequently Asked Questions About DIGIFab. DIGIFab. 2022.https://digifab.health/en-us/digifab-resources/faqs (accessed Jan 2023).
1 comment
You must be logged in to post a comment.

Great article which familiarises oneself on how to suspect and deal with Digoxin toxicity in the community