DR P. MARAZZI/SCIENCE PHOTO LIBRARY
After reading this article, you should be able to:
- Identify the symptoms of atrial fibrillation and understand how the condition is diagnosed;
- Know the different strategies for managing the condition, including the difference between rate and rhythm control;
- Understand the importance of correct anticoagulation and the advantages and disadvantages of common oral anticoagulants;
- Understand how pharmacist can help optimise patient comorbidities.
Atrial fibrillation (AF) is the most common sustained cardiac rhythm disturbance in the world, affecting close to 40 million people[1,2]. It is estimated that around 1.5 million people in England live with AF, equivalent to 2.5% of the total population[3].
Owing to the pronounced electrical and structural remodelling of the heart it induces, AF is found in 60% of patients with heart failure (HF)[4]. AF independently increases the risk of death by up to two-fold and has been linked with the development of other conditions, such as dementia[5,6]. AF impairs the emptying of blood from the atria of the heart (particularly the left atrial appendage, a small sac in the wall of the left atrium), which encourages thrombogenicity (the formation of clots), predisposing individuals to a five-fold increased risk of ischaemic stroke and systemic embolic events (SEEs)[7,8]. It is therefore not surprising that the care of AF patients costs the UK economy an estimated £2bn each year[9,10].
If detected early enough, AF can be adequately managed, allowing for the prevention or slowing of harmful effects. Besides stroke prevention, the main strategies for AF management and symptomatic relief include rhythm and rate control[11]. The former aims to restore the normal, or sinus rhythm, of the heart thereby effectively halting disease progression. However, rhythm control is not an option for all patients and rate control (i.e. the slowing down of the heart rate [HR] without correcting the rhythm), remains a common strategy, especially among older individuals[11,12].
While there are non-pharmacological interventions available (e.g. direct current cardioversion), medicines remain the cornerstone of AF treatment[11,12]. As a result, pharmacists and pharmacy technicians are involved in the diagnosis and management of the condition. Several initiatives have demonstrated that pharmacy professionals can effectively use pulse palpation or single-lead ECG devices, such as Kardia Mobile or MyDiagnostick, to help detect AF in patients attending their services, leading to improved rates of anticoagulation and substantial cost savings[13–18]. Equally, clinical pharmacists in primary and secondary care can facilitate the optimisation of oral anticoagulant (OAC) therapy among patients with known AF; for instance, by ensuring that eligible patients receive a suitable OAC, by reviewing their doses in light of renal impairment and by discontinuing inappropriate antiplatelet therapies[19,20].
The anticoagulant audit was included among the top quality criteria in the Pharmacy Quality Scheme 2021-2022, the national cardiovascular disease prevention focused programmes and the ‘Detect, protect and perfect’ initiative — in line with the target set by Public Health England, which aims to achieve a 90% anticoagulation rate among eligible patients with AF by 2029[21–25].
Signs and symptoms
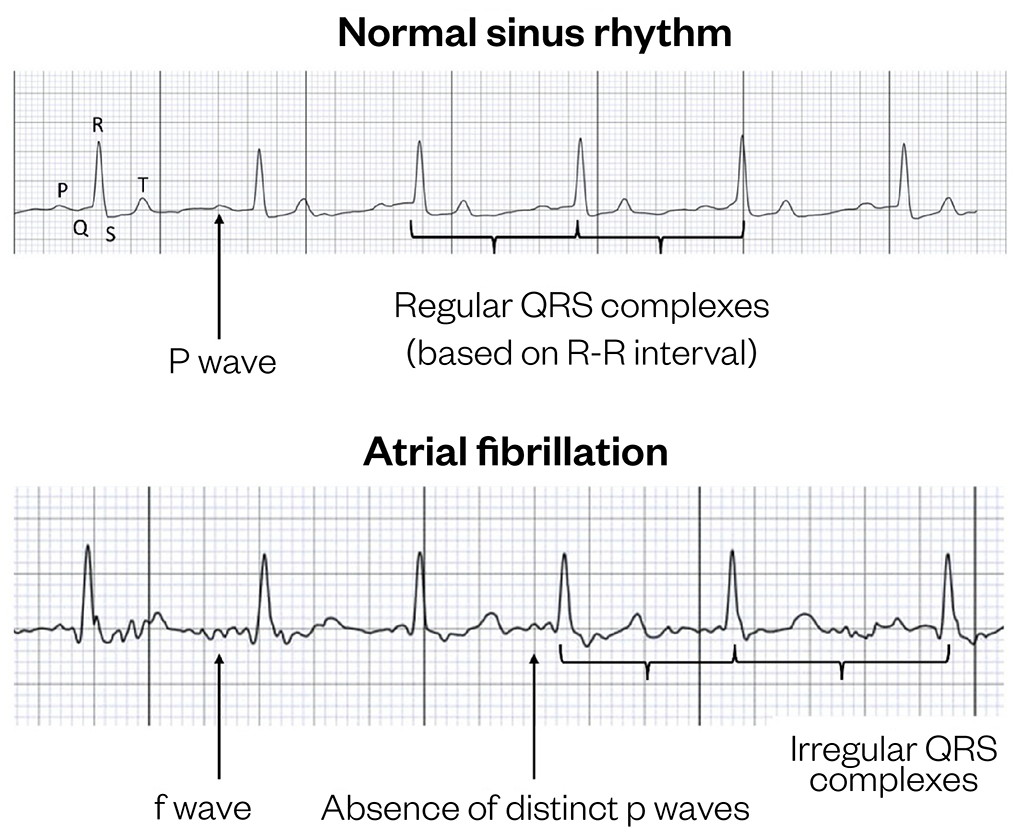
The presence of AF is characterised by continuous and usually rapid activation of the atria (≈300–600 impulses/minute), sustained by multiple depolarising ectopic foci (areas of the atria that do not normally produce electrical signals)[1,26]. This disorganised atrial activation can be seen as the absence of distinct p waves (representing atrial depolarisation) on an ECG trace. The p waves are replaced by fibrillatory (f) waves (representing very rapid atrial fibrillation, see Figure 1).
The ‘gatekeeper’ between the atria and the ventricles — the atrioventricular (AV) node — is unable to cope with multiple atrial signals and, as a result, only some of these atrial stimuli are passed onto the ventricles, producing an ‘irregularly irregular’ ventricular heart rate (HR), typically at 120–180 beats per minute (BPM). The latter is observed on an ECG as rapid and irregular QRS complexes (representing ventricular depolarisation)[1,26]. Occasionally, AF can present with a normal or slow (<60 BPM) ventricular rate (sometimes described as ‘slow AF’), typically owing to abnormalities in the sinoatrial node, the pacemaker of the heart (such as in sick sinus syndrome) and/or the AV node disease[27].
Individuals with AF can experience a range of symptoms, including breathlessness, palpitations, dizziness, fatigue, syncope and chest discomfort[11,12]. Up to 40% of patients are asymptomatic (termed ‘silent AF’) and as many as 24% may present with stroke as the first symptom[28,29]. Around 16% of AF patients experience severe or disabling symptoms, with quality of life (QoL) below the population average[11,28].
Based on the presentation and duration of symptoms, the European Society of Cardiology (ESC) distinguishes between five different patterns of AF:
- First diagnosed AF — AF that has not been diagnosed before, irrespective of its duration or symptoms;
- Paroxysmal AF (PAF) — AF that terminates spontaneously, most commonly within 48 hours, or AF that is cardioverted to normal sinus rhythm within seven days. Newly diagnosed AF that lasts <48 hours is also sometimes referred to by the National Institute for Health and Care Excellence (NICE) as ‘recent onset AF’;
- Persistent AF — AF that lasts >7 days;
- Long-standing persistent AF — AF that lasts for >12 months and is treated using a rhythm control strategy;
- Permanent AF — AF that has been accepted by the patient and clinician, and is not treated using a rhythm control strategy. This typically occurs either because a rate control strategy has been adopted instead or because rhythm control strategies have failed to maintain a sinus rhythm[11].
Adapted from Savickas (2020)[30]
Risk factors
The development of AF is influenced by both modifiable and non-modifiable risk factors, which lead to changes in electrical activity and structural remodelling of the atria (see Figure 2[30]). This slow process is mediated by a cascade involving chronic inflammation and fibrosis, some of which may occur as part of natural ageing, but are more often consistent with cardiac or non-cardiac comorbidities[31,32]. The ultimate result of long-term structural remodelling is non-uniform atria characterised by fibrotic areas and impaired electrical connections between the myocytes (i.e. cardiac muscle cells), which favours the development of AF[11,31].
The prevalence of AF increases with age; up to 10% of adults aged 65 years and over, and over 20% of those aged over 80 years, experience the condition[3,11]. Genetics play a role in AF risk, with at least 14 genomic regions implicated in the pathogenesis of AF and first-degree relatives carrying a 40% risk of developing the condition[32]. This genetic influence means that the prevalence of AF demonstrates both ethnic and geographic variation. For instance, individuals of European ancestry carry a 40% greater risk of experiencing AF compared with those of African ancestry[33]. Men are around 1.5 times more likely to develop the condition than women and this may be because they have an overall larger left atria[34–36].
This lifetime risk of AF rises from one in four people to one in three when it is accompanied by one or more modifiable risk factors, such as adverse lifestyle (e.g. alcohol intake, smoking), obesity (body mass index ≥30kg/m2) or cardiovascular disease[37]. Hypertension is the most common comorbidity, occurring in up to 80% of patients with AF[4,32,38]. Diagnoses of HF or diabetes mellitus are found in a third and fifth of AF patients, respectively[4,32].
The onset of AF is also associated with the presence of ischaemic or valvular heart diseases, chronic kidney disease and venous thromboembolism, as well as the less obvious co-factors, such as obstructive sleep apnoea, hyperthyroidism, rheumatoid arthritis, acute infections and various types of surgery[11,32].
Figure 2 shows the epidemiology and pathophysiology of AF[30].
Diagnosis
A 30-second single-lead or 12-lead ECG recording showing AF is diagnostic of the condition[11]. NICE guidance recommends performing a manual pulse palpation for individuals who present with one or more of the signs and symptoms outlined above. Where an irregular pulse is detected, the patient should be referred for a 12-lead ECG to establish the diagnosis as well as to screen them for any concomitant cardiovascular comorbidities[11,12].
Continuous 24-hour multiple-lead ambulatory ECG monitoring can help confirm a suspected PAF and is recommended by NICE if the condition remains undetected following a standard 12-lead ECG[12]. This includes patients experiencing asymptomatic PAF who are admitted to hospital with a stroke. Where symptomatic episodes of PAF are more than 24 hours apart, multiple-lead external event recorder ECG of up to 30 days can be utilised instead to detect arrhythmia and is triggered by patient symptoms[12]. Other ECG technologies, such as implantable cardiac monitors, can be used to detect AF for up to three years; however, they are often costly and reserved for specialist use only[11,12].
Following the initial diagnosis of AF, patients should be referred for transthoracic echocardiography to rule out a concomitant diagnosis of HF or other structural heart diseases. Biochemical blood tests, such as for thyroid function, blood glucose, full blood count (FBC) and urea and electrolytes are also recommended. This identifies any underlying risk factors for both AF and stroke and informs future management strategy[11,12].
Management
The ESC summarises the management of AF using the holistic ‘ABC’ pathway[39]. This has been largely embraced by the NICE guidelines and can be summarised as:
- ’A’ — Anticoagulation/avoid stroke;
- ‘B’ — Better symptom management;
- ‘C’ — Cardiovascular and comorbidity optimisation[11,12].
Anticoagulation/avoid stroke
Owing to the detrimental effects of AF-related thromboembolism, timely stroke/SEE prevention is vital, regardless of whether a rate- or rhythm-control strategy is adopted (see below). Cardioversion or catheter ablation themselves carry a small risk of stroke and some patients with AF remain at risk of recurrent arrhythmia, even once back in sinus rhythm, warranting adequate stroke prevention[11,12]. OAC therapy is the most common means of addressing the risk of stroke/SEE in patients with AF, owing to its non-invasive nature (compared with surgical interventions) and clinical effectiveness.
Before initiating OAC therapy, clinicians should consult with the patient, weighing up the benefits of stroke prevention against the risks of OAC-related bleeding[11,12]. Several validated risk stratification schemes are available to guide practitioners during such consultations. The CHA2DS2-VASc score (congestive HF, hypertension, age ≥75 years, diabetes, previous stroke, TIA or thromboembolism, vascular disease, age 65–74 years, sex category) has been validated in a variety of European and Asian cohorts and has been recommended by both NICE and the ESC for estimating an individual’s risk of stroke/SEEs[40–42].
On the basis of their CHA2DS2-VASc score, the annual risk of strokes/SEEs in a patient with AF can range from 0.2% (a score of 0) to 12.2% (a score of 9), placing patients into ‘low risk’ (a score of 0), ‘intermediate risk’ (a score of 1) or ‘high risk’ (a score of >1) categories[11,12,43]. OAC therapy should be offered to all AF patients with a score of ≥2, regardless of disease pattern or symptoms[12]. Anticoagulation should also be considered for men with a score of ≥1; female sex alone is not considered to carry a sufficiently high risk of stroke in the absence of other factors[11,12]. Stroke prevention using OAC therapy is the default option for the majority of patients, with the exception of those carrying a truly low risk of stroke (men with a CHA2DS2-VASc score of 0 or women with a CHA2DS2-VASc score of 1).
The ESC and NICE recommendations diverge when estimating OAC-related bleeding risks. The NICE panel concluded that the more contemporary Outcomes Registry for Better Informed Treatment of AF (ORBIT-AF, or simply ORBIT) score was more accurate in estimating the risk of major bleeding with OAC than the older risk stratification tools, such as HAS-BLED, which is preferred by ESC[11,12]. The ORBIT score takes into account the individual’s haemoglobin levels, age (>74 years), bleeding history, estimated glomerular filtration rate (eGFR) and the presence of concomitant anti-platelet therapy, segregating patients with AF into categories: from ‘low’ risk of major bleeding (score of 0 or 1.7 bleeds/100 patient years) to ‘high’ (score of 7 or 8.1 bleeds/100 patient years)[44].
Several risk factors included in the ORBIT and HAS-BLED scores, such as older age and hypertension, overlap with the risk factors for stroke. As such, a high bleeding risk score itself should not generally result in patient’s exclusion from OAC therapy and practitioners should instead focus on addressing modifiable risk factors for bleeding, such as stopping unnecessary antiplatelets[11,12]. This is particularly important in patients with AF who have a history of acute coronary syndromes and in whom concomitant antiplatelet therapy is indicated for up to 12 months, thereafter continuing the OAC monotherapy[11]. Work is needed to improve the anticoagulation rates in certain patient groups, such as care home residents, fewer than 50% of whom receive effective stroke prevention because of the perception of high bleeding risk[45].
Vitamin K antagonist OACs, primarily warfarin, have previously dominated the market, decreasing the risk of stroke in patients with AF by as much as 64%, while delivering a 25% reduction in all-cause mortality, compared with aspirin[46]. However, over the past few decades, prescribing trends have changed following the introduction of direct-acting OACs (DOACs), also referred to as non-vitamin K antagonist OACs (NOACs). In contrast to warfarin, which inhibits the vitamin K-dependent production of selected clotting factors without affecting those already in circulation, DOACs bind directly to either thrombin (activated factor II) or activated factor X, thus producing a rapid onset of action (see Table). A quicker offset of action makes DOACs more convenient for use in patients undergoing emergency surgery, and the smaller likelihood and/or magnitude of interactions with foods and drugs helps avoid the dietary restrictions and fluctuations in anticoagulant action seen with warfarin (the latter is affected by commonly used enzyme induces and inhibitors, such as rifampicin and clarithromycin, respectively). As such, DOACs do not require the frequent monitoring of international normalised ratio (INR) and dose alterations which have previously inconvenienced many patients receiving warfarin therapy[11,47].
Four DOACs are commercially available in the UK:
- Dabigatran (factor IIa (thrombin) inhibitor);
- Rivaroxaban;
- Apixaban;
- Edoxaban (factor Xa inhibitors)[48–51].
Additional detail on the initiation of these DOACs, including their mechanism of action can be found in ‘Supporting patients on new direct oral anticoagulant medicines’.
Compared with warfarin, DOACs are associated with a 19% reduction in the risk of stroke/SEE and a 10% reduction in all-cause mortality, while also carrying a lower risk of major bleeding and/or haemorrhagic stroke. However, this comes at the expense of an increased incidence of gastrointestinal haemorrhages[52]. NICE and ESC recommend DOACs as first-line stroke prevention in eligible patients with AF, unless they suffer from valvular AF (i.e. AF with an underlying valvular heart disease, such as severe mitral stenosis) or have other contraindications to DOAC therapy (e.g. end-stage renal disease), in which case warfarin would be a preferred choice[11,12,53].
Although important differences between DOACs exist (e.g. the frequency of administration or pharmacokinetics), in the absence of head-to-head trials, their clinical effectiveness is likely to be comparable[11,53]. NHS England and NHS Improvement have recently released a commissioning recommendation that recommended edoxaban as the first-line DOAC, followed by rivaroxaban, apixaban and dabigatran[54].
OAC therapy should be initiated as soon as possible after the diagnosis of AF and continued long-term in eligible patients. Should the patient undergo cardioversion or ablation, OAC should be continued for at least four and eight weeks after the procedure, although the long-term use of OAC therapy in such patients is determined by the presence of stroke risk factors (CHA2DS2-VASc score) rather than the rhythm status (i.e. whether or not the patient maintains a sinus rhythm)[11]. Several baseline parameters should be monitored before initiating VKAs or DOACs, including those checked at the time of diagnosis, such as the FBC (to identify anaemia or thrombocytopenia), liver function tests including the INR (to identify potential coagulopathies), body weight/height and renal function (to inform the choice of therapy and consider relevant dose adjustments)[11,55]. Renal function is particularly important to consider when optimising doses of DOACs, up to a quarter of which are known to be inappropriate, leading to greater than anticipated cardiovascular mortality[56].
In certain patients, for instance those with previous life-threatening bleeds, OAC therapy may not be an option regardless of the medicine or dose used. Left atrial appendage occlusion or exclusion, either performed surgically or using a percutaneous device, is the non-pharmacological intervention of choice in such individuals[30]. It helps reduce the formation of potential emboli in this ‘pouch’ within the atrium, decreasing the risk of stroke/SEE alongside all-cause mortality. The routine use of this procedure is compromised by the lack of adequate evidence from randomised, controlled trials and incidence of adverse events or complications, such as device embolisation and ischaemic stroke itself[11,12]. Based on evidence from historic studies, most patients also require an OAC or an oral antiplatelet for a short period of time, or longer in some cases, after the procedure in order to minimise the risk of thromboembolism[11].
The table below lists the advantages and disadvantages of common OACs[11,47,52,53][57,58].
Better symptom management
The timely management of AF-related symptoms, either by cardiac rhythm or rate control is just as crucial as appropriate stroke prevention and may improve individual’s QoL in addition to slowing down the progression of AF-mediated cardiomyopathy[11,59–61]. However, neither of the two strategies, have shown an appreciable effect on long-term clinical endpoints, such as survival[62,63]. The rate-control strategy is associated with a lower rate of hospitalisations and is overall more cost-effective than rhythm-control, making it the preferred strategy to improve patient’s symptoms, unless:
- The AF has a reversible cause;
- The AF is primarily caused by HF;
- The patient experiences a new-onset AF (usually if it has lasted <48 hours);
- The patient suffers from atrial flutter and is considered for ablation; or
- Rhythm control is more appropriate based on clinical judgement[12].
Traditionally, the target HR for most patients is 60–100 beats per minute (BPM) at rest; however, emerging evidence suggests that a wider range of <110 BPM at rest may have similar clinical outcomes unless the patient remains symptomatic[11,64]. Standard cardioselective beta-blockers (e.g. bisoprolol), rate-limiting calcium channel blockers (e.g. diltiazem) and digoxin are generally the mainstay of rate control in preference to other anti-arrhythmic medicines, because of their more favourable adverse effect profiles (see Figure 3[11,12]). The selection is guided by patient’s comorbidities and individual preferences, with some patients requiring combination therapy to relieve their symptoms[11,12]. Additional detail on dosing information and management of combination therapy for both rate and rhythm control can be found in the ESC guidelines[11].
Patients whose symptoms do not respond to the rate-control strategy or who present with severe symptoms may require control of their cardiac rhythm. Life-threatening haemodynamic instability warrants emergency electrical cardioversion (direct-current cardioversion; DCCV) to re-set the electrical circuitry of the heart and sinus rhythm. The DCCV procedure is typically performed in a hospital setting and involves a delivery of an electrical shock timed with the downstroke of the QRS complex to revert the heart back into sinus rhythm[26]. Patients with AF lasting <48 hours who do not display life-threatening symptoms should be offered electrical or pharmacological cardioversion, depending on clinical circumstances (for instance, history of failed electrical cardioversion may warrant anti-arrhythmic drugs) and resources available. Individuals whose AF lasts >48 hours should generally be offered elective electrical cardioversion and be both appropriately rate controlled and therapeutically anticoagulated for at least three weeks before the procedure[12].
Various anti-arrhythmic medicines can facilitate pharmacological cardioversion, typically by blocking the sodium, potassium or calcium currents of the myocardium and/or affecting the autonomic tone (e.g. sotalol). As with rate control, the choice of an anti-arrhythmic is primarily guided by patient’s comorbidities. For instance, amiodarone is preferred to flecainide in patients with structural heart diseases (e.g. left ventricular impairment), see Figure 4[11,12]. It is also useful in combination with electrical cardioversion, starting four weeks before the intervention and continuing for 12 months afterwards. Some anti-arrhythmics, such as propafenone, can be used as part of the ‘pill-in-the-pocket’ strategy, in which patients with PAF take their medicine when required at the onset of symptoms[11,12].
Unfortunately, up to 30% of patients who undergo electrical or pharmacological cardioversion experience recurrent AF and require further intervention[65]. Of these patients, individuals with PAF or (long-standing) persistent AF can be offered left atrial catheter ablation, usually by using radiofrequency to isolate the pulmonary veins responsible for the generation of the arrhythmia. Alternative options for rhythm control include more invasive surgical procedures (i.e. surgical ablation) with or without other rhythm-control interventions, which can double the chance of freedom from AF; however, there is an increased risk of peri-operative infections[11,66,67]. When adequate long-term rate-control or rhythm-control interventions fail to control a patient’s symptoms, a ‘pace and ablate strategy’ might be suitable. This involves the implantation of a permanent pacemaker and ablation of the AV node, electrically isolating ventricles from the fibrillating atria. Following the procedure, eligible patients should continue long-term OAC therapy as described above[11,12].
Patient consultations and comorbidity optimisation
All patients with a new diagnosis of AF should be offered a personalised package of information during the consultation that focuses on the causes and complications of AF, stroke awareness and the principles of rate and rhythm control[12]. Patients with AF often do not understand the medical terms associated with the condition; therefore, user-friendly sources of information, such as those prepared by the AF Association, will make consultations more effective[18,68,69].
Pharmacists and pharmacy professionals reviewing patients with AF in primary or secondary care are an essential resource themselves, particularly when offering evidence-based information concerning anticoagulation[20,68]. This includes explaining the balance between the risks and benefits of OAC use to encourage adherence, the posology and administration, and drug interactions, and also providing tailored advice, for instance regarding family planning or travel while on anticoagulation[70]. A recent randomised, controlled trial of older individuals receiving warfarin therapy showed that pharmacist-led intervention comprising patient education and monthly follow-ups in community pharmacy can significantly improve patient’s health-related quality of life[71].
Besides obtaining essential information about their condition and treatment, patients should be given the contact details of relevant professionals, such as their GP, cardiologist, pharmacist or arrhythmia nurse, who may be approached in case they have any further specific questions or concerns[11,12]. Charities such as the AF Association, the Heart Rhythm Alliance and the British Heart Foundation are also good networks for patients who require additional social support[72].
Patients suffering from previously outlined comorbidities should be offered appropriate treatments, for instance mineralocorticoid receptor antagonists and sodium-glucose cotransporter-2 inhibitors in patients with HF with reduced ejection fraction (<40%) or statins in eligible patients requiring a primary or secondary cardiovascular disease prevention[11,73]. The recent RACE3 trial suggested that this approach, combined with relevant changes to individual’s lifestyle and cardiac rehabilitation can improve the management of AF comorbidities and help maintain sinus rhythm[74].
Non-cardiovascular comorbidities, such as anxiety and depression, are common among patients with AF and may require signposting to counselling or clinical psychology services[11,75,76].
In addition to their ability to detect AF and facilitate the optimisation of OAC therapy, pharmacy professionals can help manage risk factors contributing to the development of AF and its consequences. A multidisciplinary initiative between primary and secondary care in London demonstrated that clinical pharmacists implementing the ‘ABC’ approach to AF management substantially improved the control of patients’ total cholesterol while simultaneously optimising their OAC therapy[20]. Several AF screening initiatives conducted by pharmacists and/or pharmacy technicians in community pharmacies helped identify cases of undiagnosed hypertension or pre-diabetes, leading to new diagnoses and improved control of blood pressure[15,55,77,78]. In one of these studies, trained pharmacy staff were also able to offer lifestyle interventions aimed at decreasing alcohol consumption, weight management and smoking cessation, all of which can contribute to better management of AF[15]. Community pharmacists can engage with patients about other cardiovascular conditions, such as cholesterol tests, BP monitoring, blood glucose level monitoring, medicines discussions and provision of healthy lifestyle advice, which may also contribute to AF care.
Monitoring and review
Annual review is recommended for all patients with AF[12]. This meeting can serve as an opportunity to re-evaluate the risk of stroke against that of bleeding, particularly if the patient was previously aged <65 years or did not have any other risk factors for stroke. Ensuring that patients are assessed for their risk of stroke every 12 months is one of the indicators for AF within the Quality and Outcomes Framework[78]. More frequent re-assessment, every four to six months, is recommended by the ESC for individuals who are initially at a low risk of stroke; however, it may be justified in those with modifiable risk factors for bleeding that might have prevented the use of OAC therapy[11].
Similarly, more frequent monitoring and review might be necessary for some patients taking DOACs. The Specialist Pharmacy Service suggests that, after the baseline monitoring, patients receiving DOAC therapy should have a review appointment after one month to identify any problems or non-adherence. Since patients with AF are eligible for the ‘New medicines service’, this appointment can be a referral opportunity to community pharmacists to encourage their adherence to therapy[79]. This should be followed by a formal annual review, which includes the monitoring of:
- FBC;
- LFTs;
- Urea and electrolytes; and
- Serum creatinine (for creatinine clearance; CrCl)[55].
Where an individual’s CrCl is <60mL/min, it is advisable to divide the value by ten and use this as a proposed monthly frequency for the monitoring of kidney function (e.g. if CrCl is 40 mL/min, monitor every four months). More frequent monitoring can be considered in the presence of acute illness, interacting medicines or for older persons[55].
Patients receiving warfarin therapy require regular INR checks to ensure that it is within typical range (2–3) and identify any factors that may have increased or decreased their readings (e.g. changes in diet or acute courses of antimicrobials). Most patients are relatively stable and can have their INR checked every one to two months. Specialised software available in primary and secondary care allows clinicians to estimate the time in therapeutic range (TTR; the percentage of time the patient’s INR is within the target range), which helps assess the level of control over warfarin therapy and should be ≥70%[11]. Where appropriate (e.g. where INR is persistently unstable), a switch to a DOAC can be considered.
Regular reviews offer an opportunity to check patient’s heart rate and blood pressure, which can identify the need to optimise the rate/rhythm control or identify/manage the underlying hypertension. Other factors, such as changes in patient’s cognitive function and adherence to therapy, should be considered alongside the INR and TTR readings.
Detailed guidance on how to switch a patient from warfarin to a DOAC and vice versa is available in the monographs of individual DOACs. Further information on the prescribing and monitoring of DOACs in different population groups has also been compiled by the European Heart Rhythm Association (EHRA) and is often included in local clinical guidelines[53].
Best practice
- Pharmacists have an important role in the multidisciplinary team when detecting AF in at-risk individuals and optimising/monitoring OAC therapy alongside the medicines used for rate or rhythm control[15,16,20];
- Bleeding risk should be managed and not automatically exclude patients from treatment[11,12];
- DOACs are the preferred option for stroke prevention in patients with AF owing to their convenience, alongside the superior reduction in mortality and haemorrhagic strokes, compared with warfarin[2,4,52];
- Rate and rhythm control helps manage symptoms and can prevent the development of AF-induced cardiomyopathy. Beta blockers, calcium channel blockers and digoxin are the primary options for rate control, depending on patient comorbidities[11,12];
- Concomitant management of risk factors for stroke and AF (e.g. smoking cessation), alongside the optimisation of comorbidities, particularly hypertension, diabetes and HF, may provide additional clinical benefits[41,74,80,81];
- Pharmacists can effectively contribute to holistic care by directly optimising the management of comorbidities/cardiovascular risk factors and through patient education[14,20,71];
- Annual review of patients with AF in primary care can help track their progress, re-evaluate the stroke/bleeding risk and identify any issues, such as medication non-adherence, while offering an opportunity to monitor relevant biochemical measures, such as FBC, LFTs and urea and electrolytes for CrCl[11,12].
- 1Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients With Atrial Fibrillation—Executive Summary. Circulation. 2006;114:700–52. doi:10.1161/circulationaha.106.177031
- 2Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. International Journal of Stroke. 2020;16:217–21. doi:10.1177/1747493019897870
- 3Atrial fibrillation prevalence estimates for local populations. Public Health England. 2015.https://www.gov.uk/government/publications/atrial-fibrillation-prevalence-estimates-for-local-populations (accessed Nov 2022).
- 4Oldgren J, Healey JS, Ezekowitz M, et al. Variations in Cause and Management of Atrial Fibrillation in a Prospective Registry of 15 400 Emergency Department Patients in 46 Countries. Circulation. 2014;129:1568–76. doi:10.1161/circulationaha.113.005451
- 5Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of Atrial Fibrillation on the Risk of Death. Circulation. 1998;98:946–52. doi:10.1161/01.cir.98.10.946
- 6Kwok CS, Loke YK, Hale R, et al. Atrial fibrillation and incidence of dementia: A systematic review and meta-analysis. Neurology. 2011;76:914–22. doi:10.1212/wnl.0b013e31820f2e38
- 7Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–8. doi:10.1161/01.str.22.8.983
- 8Frost L, Engholm G, Johnsen S, et al. Incident Thromboembolism in the Aorta and the Renal, Mesenteric, Pelvic, and Extremity Arteries After Discharge From the Hospital With a Diagnosis of Atrial Fibrillation. Arch Intern Med. 2001;161:272. doi:10.1001/archinte.161.2.272
- 9Stewart S, Murphy N, Walker A, et al. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart. 2004;90:286–92. doi:10.1136/hrt.2002.008748
- 10Harker R. NHS Expenditure. UK Parliament. 2020.https://commonslibrary.parliament.uk/research-briefings/sn00724/#:~:text=In%202018%2F19%2C%20NHS%20England,population%20and%20needs%2Dbased%20formula (accessed Nov 2022).
- 11Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). European Heart Journal. 2020;42:373–498. doi:10.1093/eurheartj/ehaa612
- 12NICE guideline [NG196] Atrial fibrillation: diagnosis and management. National Institute for Health and Care Excellence. 2021.https://www.nice.org.uk/guidance/ng196 (accessed Nov 2022).
- 13Lowres N, Neubeck L, Salkeld G, et al. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. Thromb Haemost. 2014;111:1167–76. doi:10.1160/th14-03-0231
- 14Twigg MJ, Thornley T, Scobie N. Identification of patients with atrial fibrillation in UK community pharmacy: an evaluation of a new service. Int J Clin Pharm. 2016;38:784–7. doi:10.1007/s11096-016-0303-8
- 15Antoniou S, Barnett L, Craig J, et al. P3769Rapid referral to a one-stop AF clinic following possible AF detection by community pharmacists leads to early diagnosis and appropriate anticoagulant treatment. European Heart Journal. 2019;40. doi:10.1093/eurheartj/ehz745.0619
- 16Savickas V, Stewart AJ, Rees-Roberts M, et al. Opportunistic screening for atrial fibrillation by clinical pharmacists in UK general practice during the influenza vaccination season: A cross-sectional feasibility study. PLoS Med. 2020;17:e1003197. doi:10.1371/journal.pmed.1003197
- 17Savickas V, Veale EL, Bhamra SK, et al. Pharmacists detecting atrial fibrillation in general practice: a qualitative focus group study. BJGP Open. 2020;4:bjgpopen20X101042. doi:10.3399/bjgpopen20x101042
- 18Khanbhai Z, Manning S, Hussain W. Abstract 19641: Community Pharmacist Led Atrial Fibrillation Screening Program Has the Potential to Improve Atrial Fibrillation Detection Rates and Reduce Stroke Risk. Circ 2018;134.https://www.ahajournals.org/doi/10.1161/circ.134.suppl_1.19641
- 19Pharmacist-led virtual clinics to optimise anticoagulation in AF. Public Health England. 2019.https://www.gov.uk/government/case-studies/pharmacist-led-virtual-clinics-to-optimise-anticoagulation-in-af (accessed Nov 2022).
- 20Chahal JK, Antoniou S, Earley M, et al. Preventing strokes in people with atrial fibrillation by improving ABC. BMJ Open Qual. 2019;8:e000783. doi:10.1136/bmjoq-2019-000783
- 21Pharmacy Quality Scheme, Guidance 2021/22. NHS England. 2021.https://www.cpsc.org.uk/application/files/8116/3109/5775/NHSEI_Pharmacy-Quality-Scheme-guidance-September-2021-22-Final.pdf (accessed Nov 2022).
- 22CVDPREVENT. NHS England. 2022.https://www.england.nhs.uk/ourwork/clinical-policy/cvd/cvdprevent/ (accessed Nov 2022).
- 23Explore NHS Health Check Data, Reducing heart attack and stroke. NHS Health Check. 2022.https://www.healthcheck.nhs.uk/commissioners-and-providers/data/size-of-the-prize-and-nhs-health-check-factsheet/ (accessed Nov 2022).
- 24Atrial fibrillation: Detect, protect and perfect. The AHSN Network. 2019.https://www.ahsnnetwork.com/about-academic-health-science-networks/national-programmes-priorities/atrial-fibrillation (accessed Nov 2022).
- 25Health matters: preventing cardiovascular disease. Public Health England. 2019.https://www.gov.uk/government/publications/health-matters-preventing-cardiovascular-disease/health-matters-preventing-cardiovascular-disease (accessed Nov 2022).
- 26Bunce N, Ray R. Cardiovascular disease. In: Kumar P, Clark M, eds. Kumar & Clark’s Clinical Medicine. London: : Elsevier 2017. 931–1056.
- 27Dakkak W, Doukky R. statpearls. Published Online First: 18 July 2022.http://www.ncbi.nlm.nih.gov/books/NBK470599/
- 28Freeman JV, Simon DN, Go AS, et al. Association Between Atrial Fibrillation Symptoms, Quality of Life, and Patient Outcomes. Circ: Cardiovascular Quality and Outcomes. 2015;8:393–402. doi:10.1161/circoutcomes.114.001303
- 29Sposato LA, Cipriano LE, Saposnik G, et al. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. The Lancet Neurology. 2015;14:377–87. doi:10.1016/s1474-4422(15)70027-x
- 30Savickas V. The Role of Primary Care Pharmacists in the Detection of Atrial Fibrillation. University of Kent. 2020.https://kar.kent.ac.uk/84711/ (accessed Nov 2022).
- 31Schotten U, Verheule S, Kirchhof P, et al. Pathophysiological Mechanisms of Atrial Fibrillation: A Translational Appraisal. Physiological Reviews. 2011;91:265–325. doi:10.1152/physrev.00031.2009
- 32Staerk L, Sherer JA, Ko D, et al. Atrial Fibrillation. Circ Res. 2017;120:1501–17. doi:10.1161/circresaha.117.309732
- 33Alonso A, Agarwal SK, Soliman EZ, et al. Incidence of atrial fibrillation in whites and African-Americans: The Atherosclerosis Risk in Communities (ARIC) study. American Heart Journal. 2009;158:111–7. doi:10.1016/j.ahj.2009.05.010
- 34Benjamin EJ. Independent Risk Factors for Atrial Fibrillation in a Population-Based Cohort. JAMA. 1994;271:840. doi:10.1001/jama.1994.03510350050036
- 35Vaziri SM, Larson MG, Benjamin EJ, et al. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89:724–30. doi:10.1161/01.cir.89.2.724
- 36Ko D, Rahman F, Schnabel RB, et al. Atrial fibrillation in women: epidemiology, pathophysiology, presentation, and prognosis. Nat Rev Cardiol. 2016;13:321–32. doi:10.1038/nrcardio.2016.45
- 37Staerk L, Wang B, Preis SR, et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: cohort study based on longitudinal data from the Framingham Heart Study. BMJ. 2018;:k1453. doi:10.1136/bmj.k1453
- 38Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates 11Reprints are not available. The American Journal of Cardiology. 1998;82:2N-9N. doi:10.1016/s0002-9149(98)00583-9
- 39Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol. 2017;14:627–8. doi:10.1038/nrcardio.2017.153
- 40Lip GYH, Nieuwlaat R, Pisters R, et al. Refining Clinical Risk Stratification for Predicting Stroke and Thromboembolism in Atrial Fibrillation Using a Novel Risk Factor-Based Approach. Chest. 2010;137:263–72. doi:10.1378/chest.09-1584
- 41Olesen JB, Lip GYH, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124–d124. doi:10.1136/bmj.d124
- 42Okumura K, Inoue H, Atarashi H, et al. Validation of CHA<sub>2</sub>DS<sub>2</sub>-VASc and HAS-BLED Scores in Japanese Patients With Nonvalvular Atrial Fibrillation. Circ J. 2014;78:1593–9. doi:10.1253/circj.cj-14-0144
- 43Friberg L, Rosenqvist M, Lip GYH. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. European Heart Journal. 2012;33:1500–10. doi:10.1093/eurheartj/ehr488
- 44O’Brien EC, Simon DN, Thomas LE, et al. The ORBIT bleeding score: a simple bedside score to assess bleeding risk in atrial fibrillation. Eur Heart J. 2015;:ehv476. doi:10.1093/eurheartj/ehv476
- 45Alcusky M, Lapane KL. TREATMENT OF ATRIAL FIBRILLATION IN NURSING HOMES: A PLACE FOR DIRECT ACTING ORAL ANTICOAGULANTS? Jour Nursing Home Res. 2018. doi:10.14283/jnhrs.2018.4
- 46Hart RG, Pearce LA, Aguilar MI. Meta-analysis: Antithrombotic Therapy to Prevent Stroke in Patients Who Have Nonvalvular Atrial Fibrillation. Ann Intern Med. 2007;146:857. doi:10.7326/0003-4819-146-12-200706190-00007
- 47Mekaj A, Mekaj Y, Duci S, et al. New oral anticoagulants: their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. TCRM. 2015;:967. doi:10.2147/tcrm.s84210
- 48Dabigatran etexilate. BNF. https://bnf.nice.org.uk/drugs/dabigatran-etexilate/ (accessed Nov 2022).
- 49Rivaroxaban. BNF. https://bnf.nice.org.uk/drugs/rivaroxaban/ (accessed Nov 2022).
- 50Apixaban. BNF. https://bnf.nice.org.uk/drugs/apixaban/ (accessed Nov 2022).
- 51Edoxaban. BNF. https://bnf.nice.org.uk/drugs/edoxaban/ (accessed Nov 2022).
- 52Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. The Lancet. 2014;383:955–62. doi:10.1016/s0140-6736(13)62343-0
- 53Steffel J, Collins R, Antz M, et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. EP Europace. 2021;23:1612–76. doi:10.1093/europace/euab065
- 54Operational note: Commissioning recommendations for national procurement for DOACs. NHS England. 2022.https://www.england.nhs.uk/wp-content/uploads/2022/01/B1279-national-procurement-for-DOACs-commissioning-recommendations-v1.pdf (accessed Nov 2022).
- 55DOACs (Direct Oral Anticoagulants) monitoring. Specialist Pharmacy Service. 2021.https://www.sps.nhs.uk/monitorings/doacs-direct-oral-anticoagulants-monitoring (accessed Nov 2022).
- 56Camm AJ, Cools F, Virdone S, et al. Mortality in Patients With Atrial Fibrillation Receiving Nonrecommended Doses of Direct Oral Anticoagulants. Journal of the American College of Cardiology. 2020;76:1425–36. doi:10.1016/j.jacc.2020.07.045
- 57vitamin K1 (phytonadione) (Rx). Medscape. 2022.https://reference.staging.medscape.com/drug/vitamin-k-mephyton-vitamin-k1-phytonadione-344424 (accessed Nov 2022).
- 58SAVAYSA (edoxaban) tablets. US Food and Drug Administration. 2015.https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206316lbl.pdf (accessed Nov 2022).
- 59Peters KG, Kienzle MG. Severe cardiomyopathy due to chronic rapidly conducted atrial fibrillation: complete recovery after restoration of sinus rhythm. The American Journal of Medicine. 1988;85:242–4. doi:10.1016/s0002-9343(88)80352-8
- 60Van Gelder IC, Crijns HJGM, Blanksma PK, et al. Time course of hemodynamic changes and improvement of exercise tolerance after cardioversion of chronic atrial fibrillation unassociated with cardiac valve disease. The American Journal of Cardiology. 1993;72:560–6. doi:10.1016/0002-9149(93)90352-d
- 61Camm AJ, Savelieva I, Lip GYH, et al. Rate control in the medical management of atrial fibrillation. Heart. 2007;93:35–8. doi:10.1136/hrt.2006.099903
- 62HAGENS V, VERMEULEN K, TENVERGERT E, et al. Rate control is more cost-effective than rhythm control for patients with persistent atrial fibrillation ? results from the RAte Control versus Electrical cardioversion (RACE) study. European Heart Journal. 2004;25:1542–9. doi:10.1016/j.ehj.2004.06.020
- 63A Comparison of Rate Control and Rhythm Control in Patients with Atrial Fibrillation. N Engl J Med. 2002;347:1825–33. doi:10.1056/nejmoa021328
- 64Van Gelder IC, Groenveld HF, Crijns HJGM, et al. Lenient versus Strict Rate Control in Patients with Atrial Fibrillation. N Engl J Med. 2010;362:1363–73. doi:10.1056/nejmoa1001337
- 65Crijns HJGM, Weijs B, Fairley A-M, et al. Contemporary real life cardioversion of atrial fibrillation: Results from the multinational RHYTHM-AF study. International Journal of Cardiology. 2014;172:588–94. doi:10.1016/j.ijcard.2014.01.099
- 66Huffman MD, Karmali KN, Berendsen MA, et al. Concomitant atrial fibrillation surgery for people undergoing cardiac surgery. Cochrane Database of Systematic Reviews. 2016;2020. doi:10.1002/14651858.cd011814.pub2
- 67McClure GR, Belley-Cote EP, Jaffer IH, et al. Surgical ablation of atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. EP Europace. 2017;20:1442–50. doi:10.1093/europace/eux336
- 68Alghadeer SM, Alzahrani AA, Alalayet WY, et al. <p>Anticoagulation Control of Warfarin in Pharmacist-Led Clinics Versus Physician-Led Clinics: A Prospective Observational Study</p> RMHP. 2020;Volume 13:1175–9. doi:10.2147/rmhp.s248222
- 69AF Information & Advice For Patients . Atrial Fibrilllation Association. 2022.https://www.heartrhythmalliance.org/afa/uk/for-patients (accessed Nov 2022).
- 70NICE guideline [NG158] Venous thromboembolic diseases: diagnosis, management and thrombophilia testing . National Institute for Health and Care Excellence. 2020.https://www.nice.org.uk/Guidance/NG158 (accessed Nov 2022).
- 71Falamić S, Lucijanić M, Ortner-Hadžiabdić M, et al. Pharmacists’ interventions improve health-related quality of life of rural older person on warfarin: a randomized controlled trial. Sci Rep. 2021;11. doi:10.1038/s41598-021-01394-0
- 72Mairesse GH, Moran P, Van Gelder IC, et al. Screening for atrial fibrillation: a European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLAECE). EP Europace. 2017;19:1589–623. doi:10.1093/europace/eux177
- 73McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. European Heart Journal. 2021;42:3599–726. doi:10.1093/eurheartj/ehab368
- 74Rienstra M, Hobbelt AH, Alings M, et al. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial. European Heart Journal. 2018;39:2987–96. doi:10.1093/eurheartj/ehx739
- 75Thrall G, Lip GYH, Carroll D, et al. Depression, Anxiety, and Quality of Life in Patients With Atrial Fibrillation. Chest. 2007;132:1259–64. doi:10.1378/chest.07-0036
- 76Polikandrioti M, Koutelekos I, Vasilopoulos G, et al. Anxiety and Depression in Patients with Permanent Atrial Fibrillation: Prevalence and Associated Factors. Cardiology Research and Practice. 2018;2018:1–9. doi:10.1155/2018/7408129
- 77Sandhu RK, Dolovich L, Deif B, et al. High prevalence of modifiable stroke risk factors identified in a pharmacy-based screening programme. Open Heart. 2016;3:e000515. doi:10.1136/openhrt-2016-000515
- 78Quality and Outcomes Framework guidance for 2021/22. NHS England. 2021.https://www.england.nhs.uk/wp-content/uploads/2021/03/B0456-update-on-quality-outcomes-framework-changes-for-21-22-.pdf (accessed Oct 2022).
- 79Advanced Service Specification – NHS New Medicine Service (NMS). NHS England. 2021.https://www.england.nhs.uk/wp-content/uploads/2021/10/B0936-service-specification-nhs-nms-advanced-service.pdf (accessed Oct 2022).