Shutterstock.com
Learning objectives:
After completing this module, individuals should be able to:
- Understand the impact of inflammatory bowel disease on patient quality of life;
- Appreciate the difference between inflammatory bowel disease and irritable bowel syndrome;
- Distinguish between symptoms and presentation of Crohn’s disease and ulcerative colitis;
- Understand how Crohn’s disease and ulcerative colitis are diagnosed.
Inflammatory bowel disease (IBD) is a term that describes the intestinal disorders Crohn’s disease (CD) and ulcerative colitis (UC). These disorders are characterised by chronic inflammation of the gastrointestinal tract[1]. They follow a relapsing and remitting course that can be unpredictable[1].
IBD is a life-long condition associated with considerable ongoing morbidity and can affect an individual’s social and psychological wellbeing, particularly if their disease is poorly controlled[2]. The management of IBD is associated with high healthcare costs and requires a multidisciplinary approach across primary and secondary care[3].
Most patients are diagnosed when they are relatively young, significantly affecting their lives in many ways. For example:
- Clinical symptoms (e.g. fatigue, urgent need for toilet, pain);
- Psychological issues (e.g. loss of self-esteem & increased anxiety);
- Loss of education;
- Difficulties with gaining employment and insurance;
- Personal relationship issues and social functioning;
- Growth failure or retarded sexual development;
- Complications of surgery (e.g. infection, stoma, intestinal failure);
- Medication — long-term maintenance therapy, adherence, side effects, infection, intolerance and/or loss of response, access to high cost and off-label medications;
- Secondary health problems (e.g. osteoporosis, anaemia);
- Precautions with conception, pregnancy and breast feeding including genetic associations[3].
UC and CD are related diseases but there are contrasting features with regards to the clinical presentation, the site of involvement and extent of inflammation across the bowel wall[4].
IBD is not the same as irritable bowel syndrome (IBS), which is a common condition that causes symptoms such as bloating, abdominal pain, constipation or diarrhoea[5]. Although they share some symptoms and are not mutually exclusive, they are two distinct conditions that require different approaches to management. For a visual guide to IBS, see here.
This article, the first in a two-part series, focuses on the diagnosis of IBD. Part two will focus on the treatment options of underlying disease, its complications and their implications for patient health and wellbeing.
Epidemiology
Over 6.8 million people are estimated to be living with IBD worldwide, with 300,000–500,000 people currently living with a diagnosis of IBD in the UK[6–8]. A 2018 population-based cohort study published in 2020 estimated UK point prevalence as 276 cases of CD per 100,000 people and 397 cases of UC per 100,000[9].
The highest occurrence of IBD is seen in developed countries in North America and Europe, affecting up to 0.5% of the general population[10]. Although the incidence of IBD is stabilising in western countries, prevalence remains high owing to improved survival rates and longer life expectancy[11].
Up to 90% of people with UC will experience one or more relapses after the first episode; early relapse or active disease in the first two years following diagnosis is associated with a poorer outcome[3].
Age
IBD most commonly occurs between the ages of 15 and 40 years, although it can occur at any age, with 15% of cases diagnosed in individuals aged over 60 years[3,12]. Up to one third of patients with CD are diagnosed before the age of 21 years[13]. UC is commonly diagnosed in late adolescence or early adulthood, with reports potentially suggesting a second incidence peak in 60–69 year olds[14].
Race and ethnicity
Prevalence among individuals of African American or Hispanic ethnicity appear to have a lower incidence of IBD than white populations[15].
Incidence and prevalence in Asian countries has increased in recent years owing to better diagnosis as a result of rapid urbanisation and industrialisation. This is likely to have relevance to the UK and its diverse population[16].
Jewish people have an increased risk of IBD; this is higher among Ashkenazi, American and European populations compared to those living in Israel[17,18].
Pathophysiology
The pathogenesis of IBD remains poorly understood but is thought to arise from dysregulated immune responses and an alteration in the composition and function of gut bacteria and/or their metabolites[19]. While no specific pathogen has been implicated as a causal factor, studies have shown an association with Escherichia coli, Salmonella, Campylobacter and mycobacteria[3,5].
In people with IBD, trigger factors — such as stress, infection, use of non-steroidal anti-inflammatory drugs (NSAIDs), diet and non-adherence to medication — typically cause a severe, prolonged and inappropriate inflammatory response in the gastro-intestinal tract[20]. The ongoing inflammatory response leads to an alteration in the normal architecture of the digestive tract. Genetically susceptible individuals seem unable to down-regulate immune or antigen non-specific inflammatory responses. Some studies have highlighted that the inflammation is characterised by increased activity of effector lymphocytes and pro-inflammatory cytokines that override normal control mechanisms. Others, however, have suggested that IBD may result from a primary failure of regulatory lymphocytes and cytokines, such as interleukin (IL)-10 and transforming growth factor-β, to control inflammation and effector pathways. In CD, it is also thought that T-cells are resistant to apoptosis after inactivation[1,17,21]. Figure 1 summarises the pathogenesis of IBD.
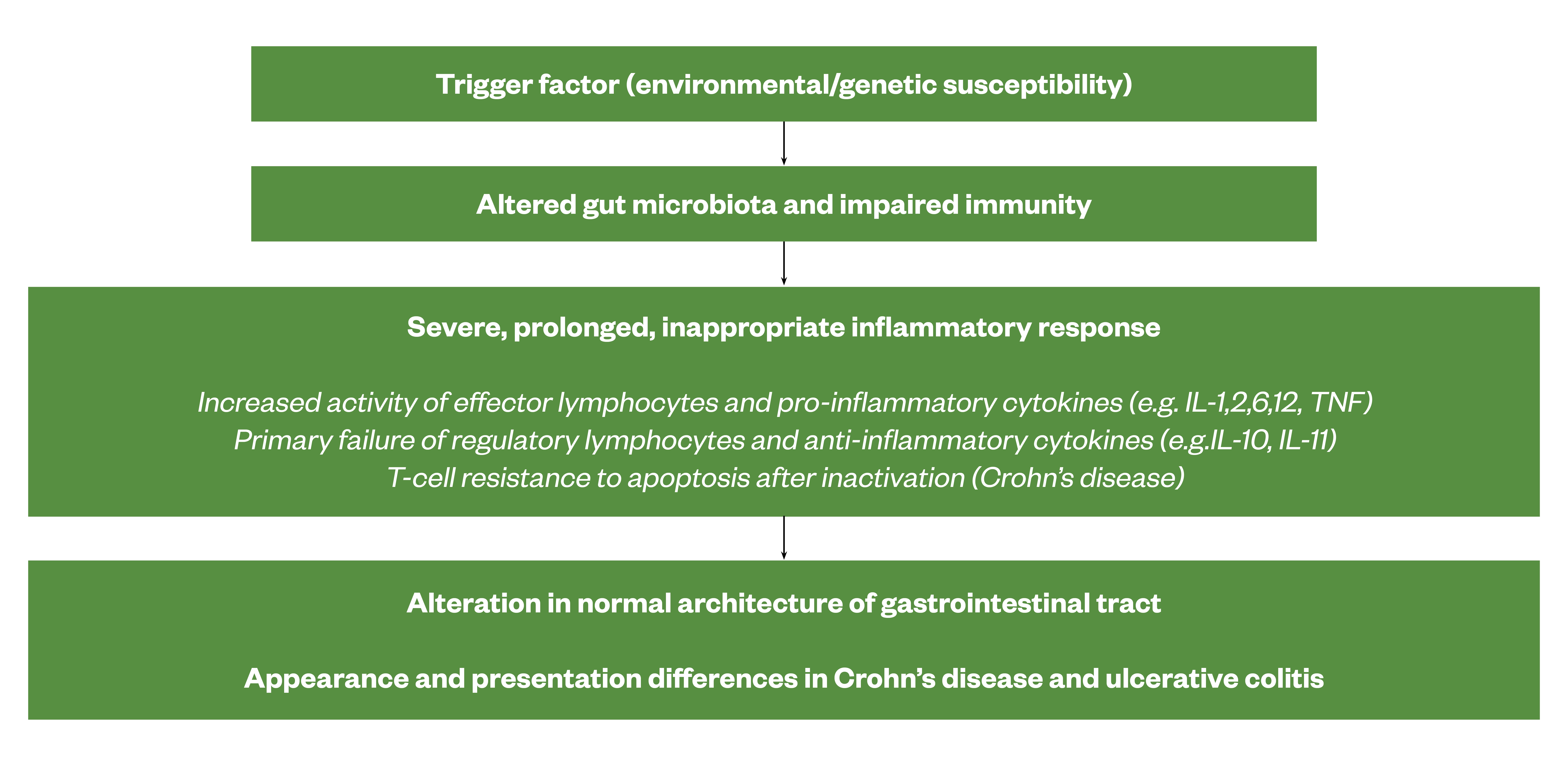
Sources: [1,17,21]
Risk factors
The precise aetiology of IBD is unknown although its development and progression are multifactorial, with genetic, environmental and an altered gut microbiota thought to be involved[22].
Genetic factors
A genetic predisposition to IBD is well established. There is a high concordance rate for CD among monozygotic twins and a 3- to 20-fold increased risk of IBD in first-degree relatives of patients with IBD[23,24]. Disease location (e.g. colonic versus ileal) and clinical phenotype (e.g. fibrostenotic, fistulising) in CD appear to have a heritable pattern[25]. The offspring of affected parents may have earlier onset of disease and increased severity at presentation[26]. Having a positive family history remains the strongest risk factor for developing IBD[27].
Genetic studies have enabled us to identify specific pathways that are important in the pathogenesis of the disease[28]. Of the genes identified to date, NOD2/CARD15 confers the greatest risk of developing CD with major histocompatibility complex (MHC) locus human leukocyte antigen (HLA) class II alleles having the most consistent association with UC[28]. Adaptive immune genes that regulate IL-12, IL-17 and IL-23 receptor pathway have also been implicated in IBD risk[29,30].
Diet
Diets high in animal fats, sugar and processed foods and low in fruit and vegetables can increase risk of developing IBD or experiencing a relapse[31]. Vitamin D deficiency is common in IBD patients and emerging data suggest that it might have a role in its pathogenesis and course[3,17]. It is unclear if obesity is associated with increased risk; however, accumulation of intra-abdominal fat may contribute to mucosal inflammation[32]. Breastfeeding in infancy can protect against the development of CD and UC[33].
Smoking
People who smoke are twice as likely to develop CD than non-smokers[34]. Smoking worsens the clinical course of the disease and increases the risk of relapse and the need for surgery[34]. UC is more common in non-smokers and those who have recently quit, particularly within the first 2–5 years following cessation[34]. All IBD patients should be encouraged to stop smoking owing to the wider health benefits; UC patients should be made aware there is an increased risk of a flare up after quitting[3].
Medicines
NSAIDs may precipitate a relapse in up to one third of users. It is thought this may result from the non-selective inhibition of cyclo-oxygenase (COX) enzymes. Higher doses and longer duration of use confer increased risk[17].
Antibiotics may also precipitate a relapse in disease due to a change in the enteric microflora[35]. In Western populations, the use of antibiotics in childhood has been linked with increased IBD risk, more so for CD than UC[36].
Hormone replacement therapy and oral contraceptives may increase the risk of IBD, possibly caused by vascular changes or oestrogen enhancing the inflammatory response[15].
There has been much debate about the link between IBD and the measles vaccine or combined measles, mumps and rubella (MMR) immunisation; however, there is no proven correlation[37].
Appendicectomy
Evidence suggests that appendicectomy is associated with a lower risk of UC, whereas the opposite is true for CD[38]. However, this may owe to a misdiagnosis in patients with incipient CD where the presenting symptoms/signs can be similar between the two conditions[39].
Stress
Observational studies suggest an association between major life stressors, anxiety and depression and an increased risk of IBD[40,41]. It is thought that stress activates inflammatory mediators at enteric nerve endings in the gut wall[40]. In those with established disease, depression and anxiety is linked with relapse, hospitalisation, surgery and reduced quality of life[17].
Disease location
UC only affects the large bowel (colon) and is characterised by continuous mucosal inflammation beginning in the rectum and extending proximally[42]. Inflammation is mucosal and does not extend transmurally (unlike CD). Different terms are used to describe the degree of involvement:
- Proctitis — rectum only;
- Left sided colitis — distal to the splenic flexure;
- Extensive colitis — any extent proximal to the splenic flexure;
- Pancolitis — involvement of the entire colon[42].
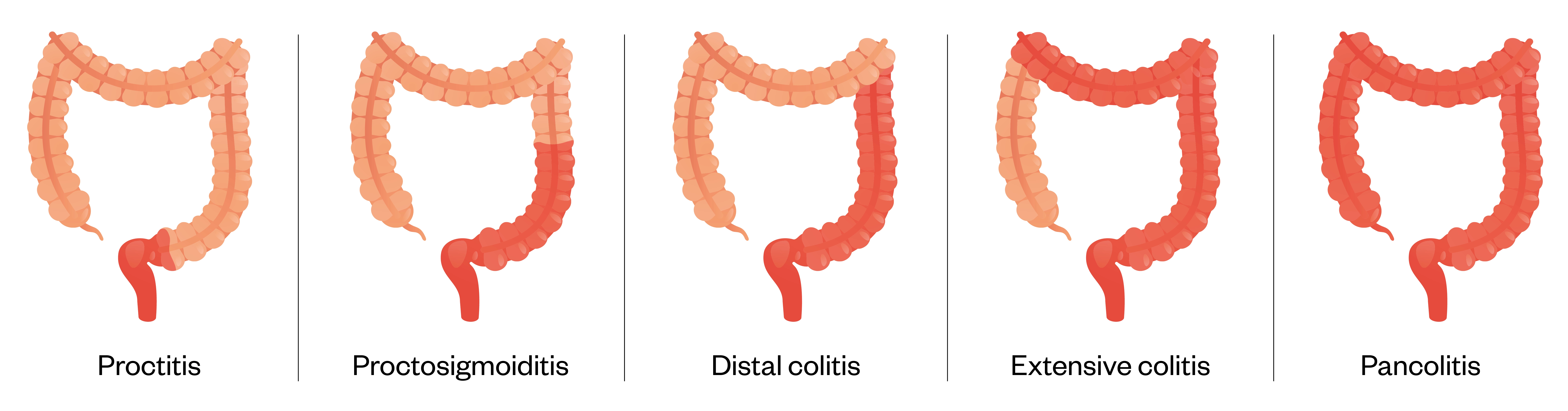
At first presentation, 20% of patients are diagnosed with extensive colitis[43]. Rectal sparing occurs in >3% of patients[3]. The reasons for differing disease presentation are unknown[1].
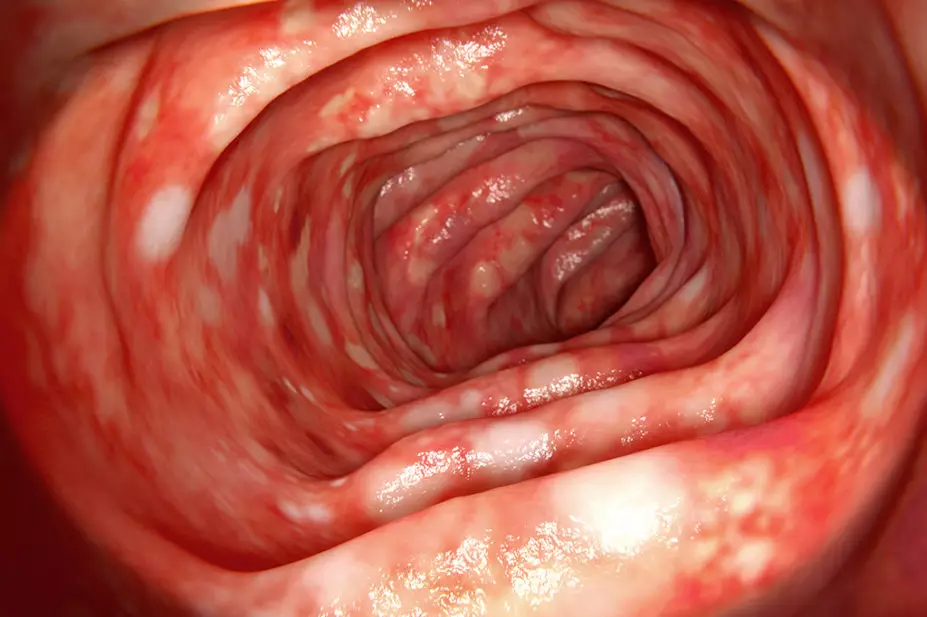
CD can affect any part of the gastrointestinal tract from mouth to the perianal region, but most commonly involves the ileum and proximal colon[44]. Around one third of patients will have perianal disease[3]. Mucosal biopsies show patchy inflammation or skip lesions (segments of normal-appearing bowel interrupted by areas of disease) extending through the bowel wall to the serosal surface[3]. Inflammation and ulceration are transmural and may lead to fibrosis, strictures and an obstructive clinical presentation, together with the formation of fistulae[45]. The areas of the bowel affected may be thickened and narrowed — this is more likely to cause symptoms in the small intestine which has a narrower diameter than the colon[3].
Chronic inflammation in the gastrointestinal tract triggers cellular events that promote malignant transformation of normal epithelial cells. This increases the risk of dysplasia and progression to cancer[46].
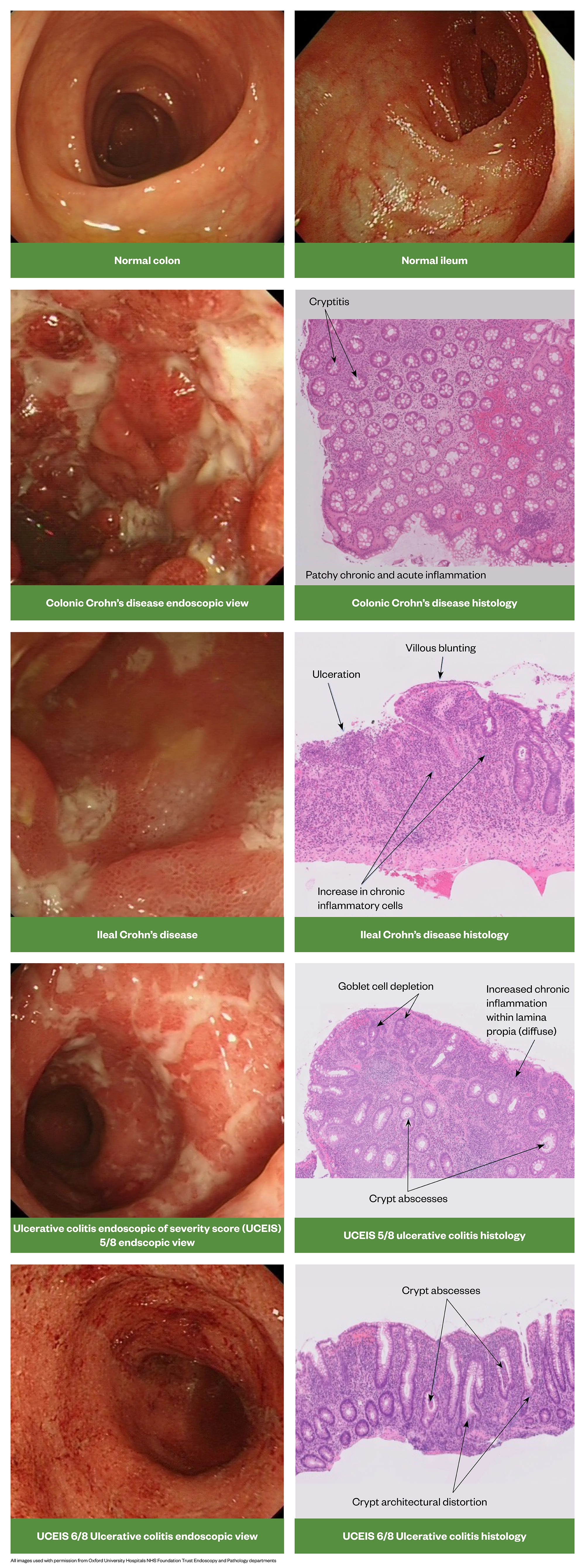
IBD-unclassified (IBD-U) — also termed intermediate colitis — is diagnosed in 5–15% of cases and is more common in children[3]. Here it is not possible to distinguish between CD or UC despite endoscopy, radiology and histology.
Other types of colitis include microscopic colitis (which can be differentiated into lymphocytic colitis or collagenous colitis)[47]. The main feature is watery diarrhoea in the presence of a normal colonoscopy, and chronic inflammation in the absence of crypt architectural distortion on mucosal biopsies[47]. Drugs such as NSAIDs and proton pump inhibitors (PPIs) are implicated as the cause in up to 50% of cases of microscopic colitis[48].
Diversion colitis is inflammation that occurs in approximately 91% of patients with a defunctioned colon, resulting in mucus discharge[49]. Pseudomembranous colitis is caused by Clostridioides difficile, usually after prolonged or multiple courses of antibiotics[50]. The use of PPIs predisposes patients to C. difficile infection. It is diagnosed by sigmoidoscopy and detection of C. difficile toxin in the stool.
Clinical features — signs and symptoms
Ulcerative colitis
Typical symptoms of active disease or relapse include bloody diarrhoea (the most predominant symptom) with mucus, urgency and frequency[8]. Crampy abdominal pain is associated with (and relieved by) an urge to defaecate.
Most patients with UC will have mild disease at first presentation, with 15% presenting with severe disease[51]. Disease severity is important in guiding clinical management and predicting outcomes.
Crohn’s disease
The predominant symptoms in CD are diarrhoea (often non-bloody but may contain some blood and mucus), abdominal pain, fatigue and weight loss[7]. Around 50-80% of patients will require surgery for indications including strictures with obstructive symptoms, perianal disease (i.e. formation of fissures, fistulae and abscesses), intestinal perforation, or failure of medical therapy[52].
Fistulae develop in around 25–33% of people with CD[53]. Fistulae are much less common in people with UC as the inflammation does not penetrate the thickness of the bowel wall, unlike in CD[34]. In CD, examples of fistulae include:
- Perianal (anal canal to skin surface) — this is the most common;
- Entero-vesical/colo-vesical (bowel to bladder);
- Entero-vaginal (bowel to vagina);
- Recto-vaginal fistula (rectum to vagina);
- Entero-cutaneous (bowel to skin in areas other than near the anus);
- Entero-enteric or entero-colic (linking different parts of the bowel or intestine together, bypassing a section in between)[34].
Figure 13 illustrates perianal fistulae. Perianal fistulas are a disabling manifestation and source of morbidity for CD patients, associated with pain, discharge and risk of infection. Treatment requires a combined surgical and medical approach[54].
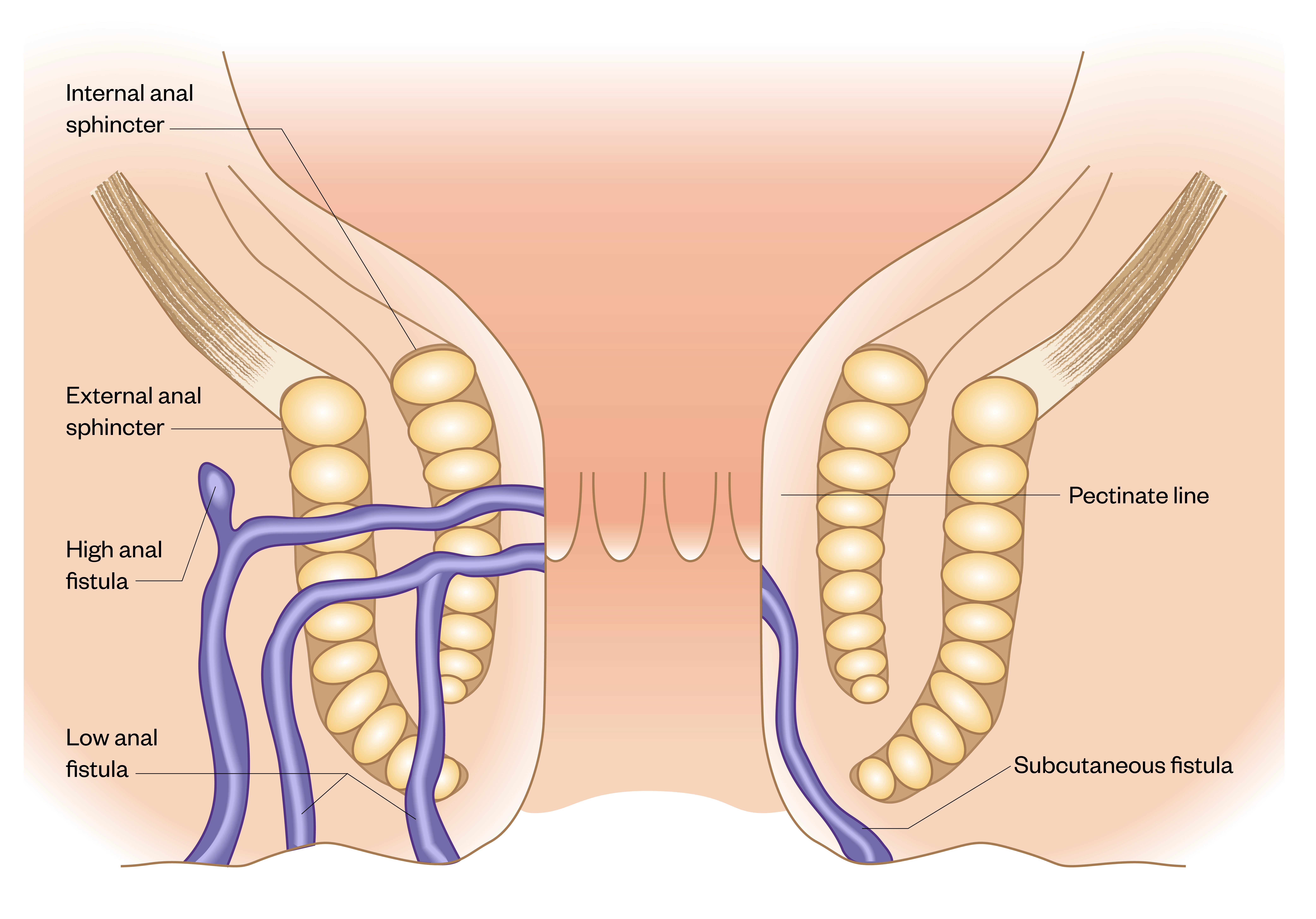
Source: [54]
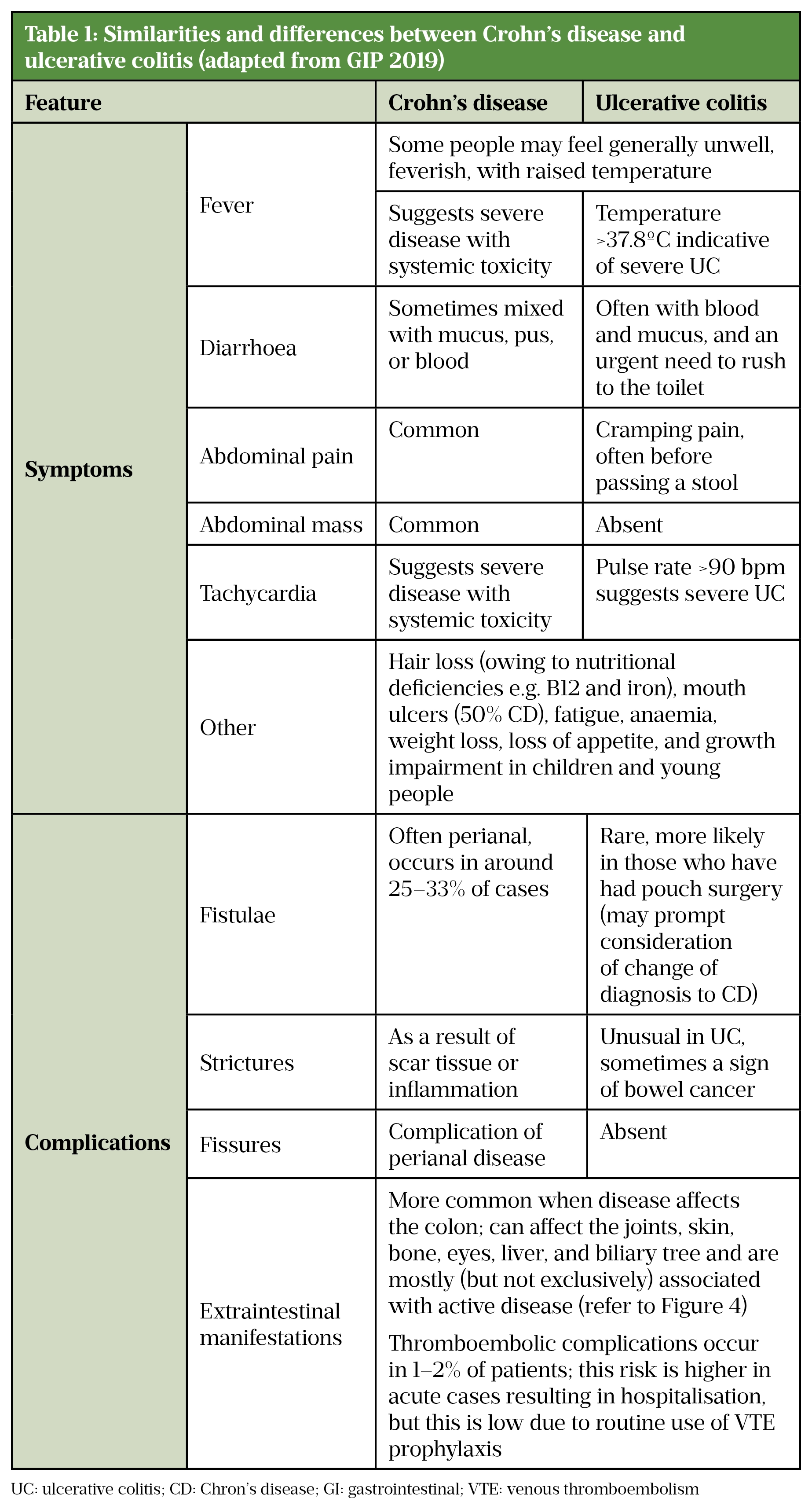
The clinical similarities and differences between CD and UC are highlighted in Table 1.
Sources: [7,8,10,13,43,55]
Extraintestinal features
Up to 50% of patients with IBD experience at least one extra-intestinal complication affecting the joints, skin, bone, eyes, liver and biliary tree, and are mostly associated with active disease[51]. Hospitalised IBD patients are at risk of death from pulmonary embolism. However, the risk is low due to routine use of pharmacological thromboembolic prophylaxis in hospitalised patients[56].
Figure 14 highlights some of the extra-intestinal features of IBD such as pyoderma gangrenosum), discrete pustule that develops into a non-infectious ulcer manifesting in 1 – 3% of IBD patients[1].
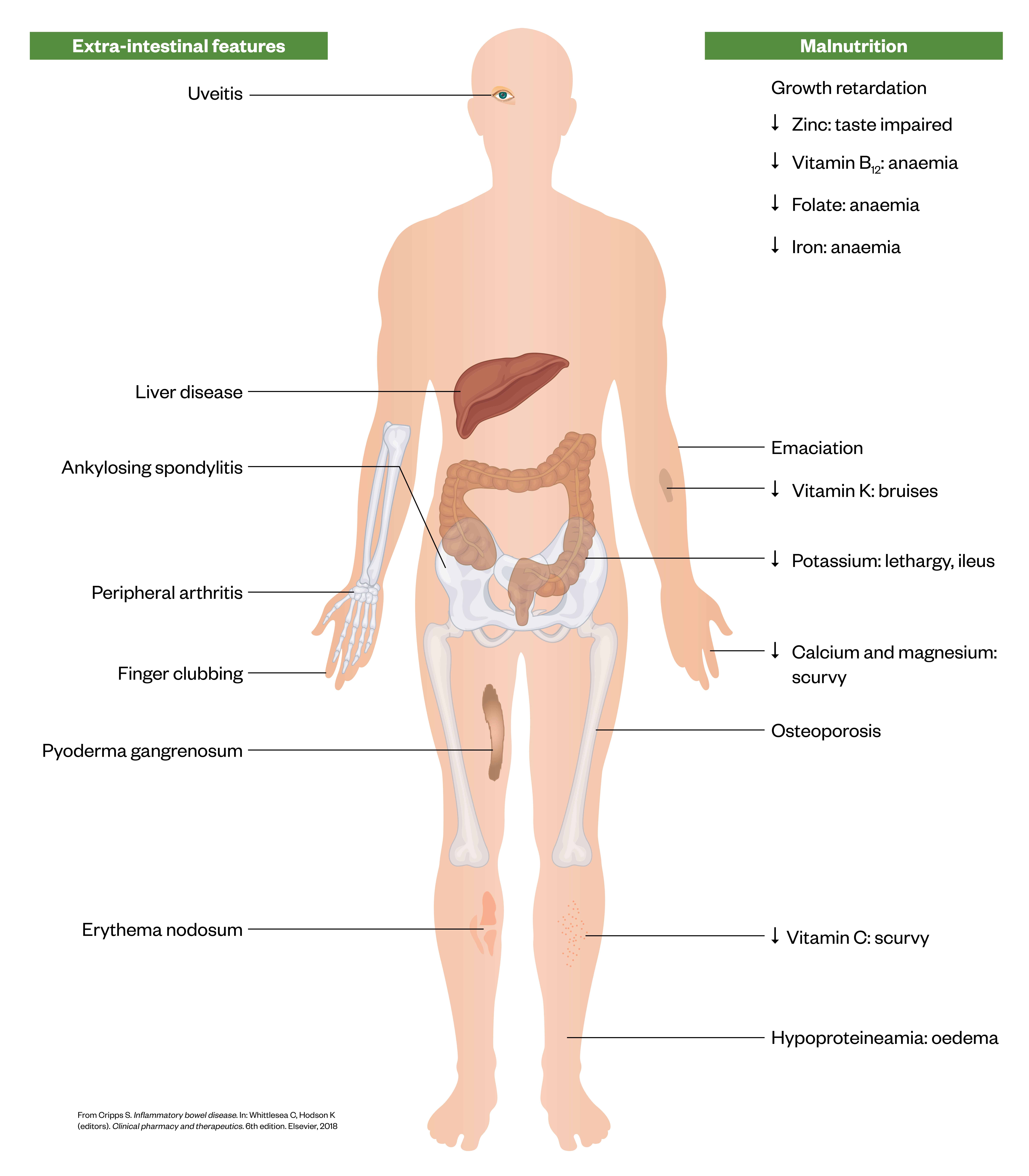
Source:[1]
Complications
In UC, complications include toxic dilatation of the colon and the risk of perforation, which although rare, is associated with a 50% mortality rate[57,58].
In some cases, large or repeated surgical resections of inflamed bowel (with or without stoma formation) are required [51]. This can lead to short bowel syndrome, which may require medical management to ensure adequate absorption of nutrients, fluid and drugs. Life-long nutritional support in the form of parenteral nutrition may be needed[59].
IBD patients are at an increased risk of solid tumours, skin and haematological malignancies; choice of treatment may be affected if there is a previous history of malignancy[60].
There is an increased risk of colorectal cancer in patients with colonic disease (apart from isolated proctitis) and scheduled colonoscopic surveillance is recommended[61].
The risk of leukaemia in UC and of lymphoma in CD is greater than the national averages[60]. These risks return to baseline if inflammation in the gut is treated. Early disease onset, male gender, and age >65 years are risk factors for haematological malignancies in IBD patients[60].
It is unclear whether IBD is an independent risk factor for melanoma; however, it is known to increase the risk of non-melanoma skin cancers such as squamous-cell carcinoma[60].
Other complications of IBD include anaemia (iron, folate and/or B12 deficiency) and osteoporosis[55]. Depression and anxiety are also common, partly owing to many patients being diagnosed with a chronic relapsing-remitting disease at a key stage of life[55].
Diagnosis
The combination of clinical signs and symptoms, together with laboratory tests (see Table 2), endoscopic, radiological, and histological parameters establishes the diagnosis, and identifies disease relapse and response to treatment[3]. Objective assessment of disease activity is important for guiding therapy as part of a “treat to target” strategy[62].
Differential diagnoses of IBD include carcinoma, infection, drug-induced colitis, ischaemia, radiation damage, irritable bowel syndrome, coeliac disease, lactose intolerance and diverticulitis[63].
A full patient history should include recent travel, medication (such as recent use of antibiotics or NSAIDS), sexual and vaccination history (as appropriate), and identification of potential risk factors such as smoking, family history and recent gastroenteritis. Nutritional assessment carried out by a registered dietitian or relevant healthcare professional is also important in diagnosis and subsequent management.
Laboratory tests
Table 2 summarises some of the main tests routinely used to support the diagnosis and assessment of disease activity of IBD and its complications[64].
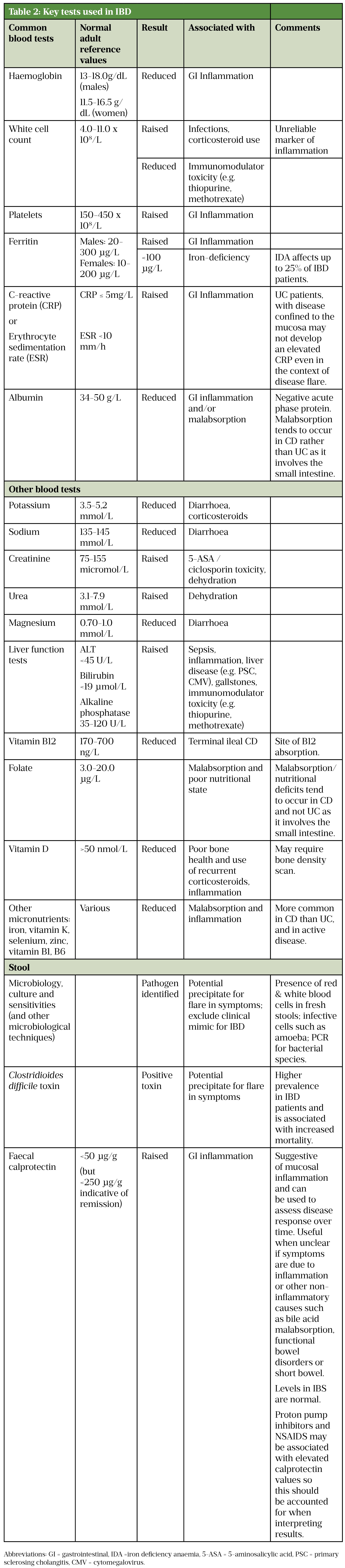
Sources: [3,7,62,64]
Histopathology
There are a number of important histopathological differences between CD and UC. Figure 15 provides a useful summary .
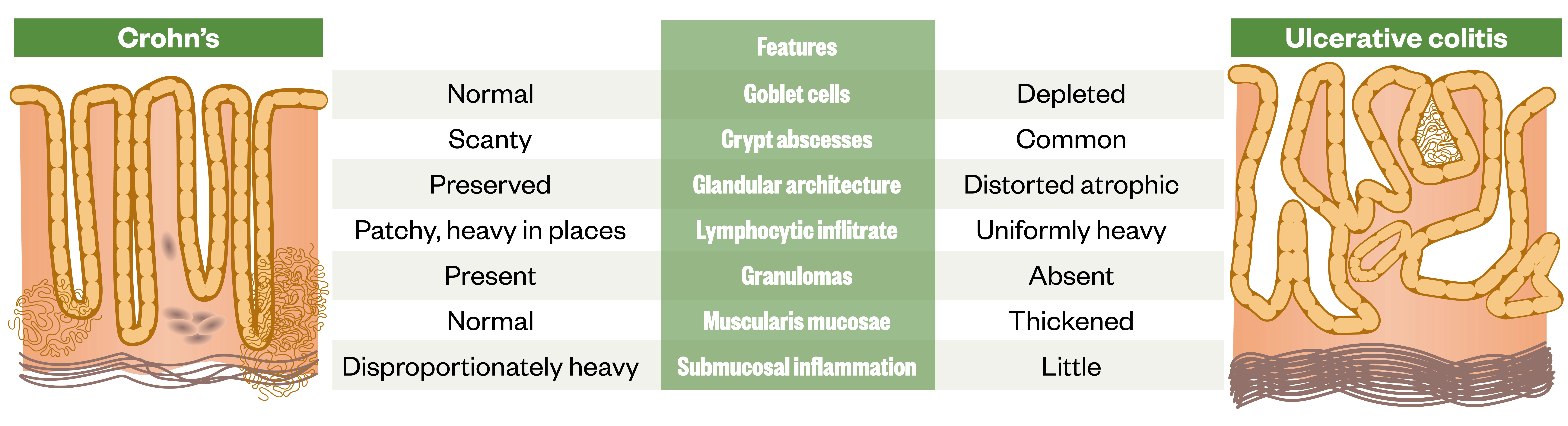
Source: [1]
Clinical assessment tools
Crohn’s disease
In clinical trials, the Crohn’s Disease Activity Index (CDAI)[65] or the Harvey–Bradshaw Index (HBI)[66] (see Table 3) are the tools most often used to define remission in adults with Crohn’s disease[67]. However, in clinical practice the CDAI is rarely used as it needs to be measured prospectively and is time consuming. Additionally, it is also not validated to be used post-operatively. The HBI evaluates stool frequency, pain and other clinical features, including extra-intestinal complications, and is increasingly used to guide selection and measure response of biologic therapy [13,43].
A CDAI score of <150 or an HBI of 4 or below suggests the patient is in remission, whereas a CDAI of ≥300, or an HBI of >8 indicates severe active disease[3]. When using these scores, consideration should be made for any physical, sensory or learning disabilities or communication difficulties that could impact patient responses and adjusted as appropriate.
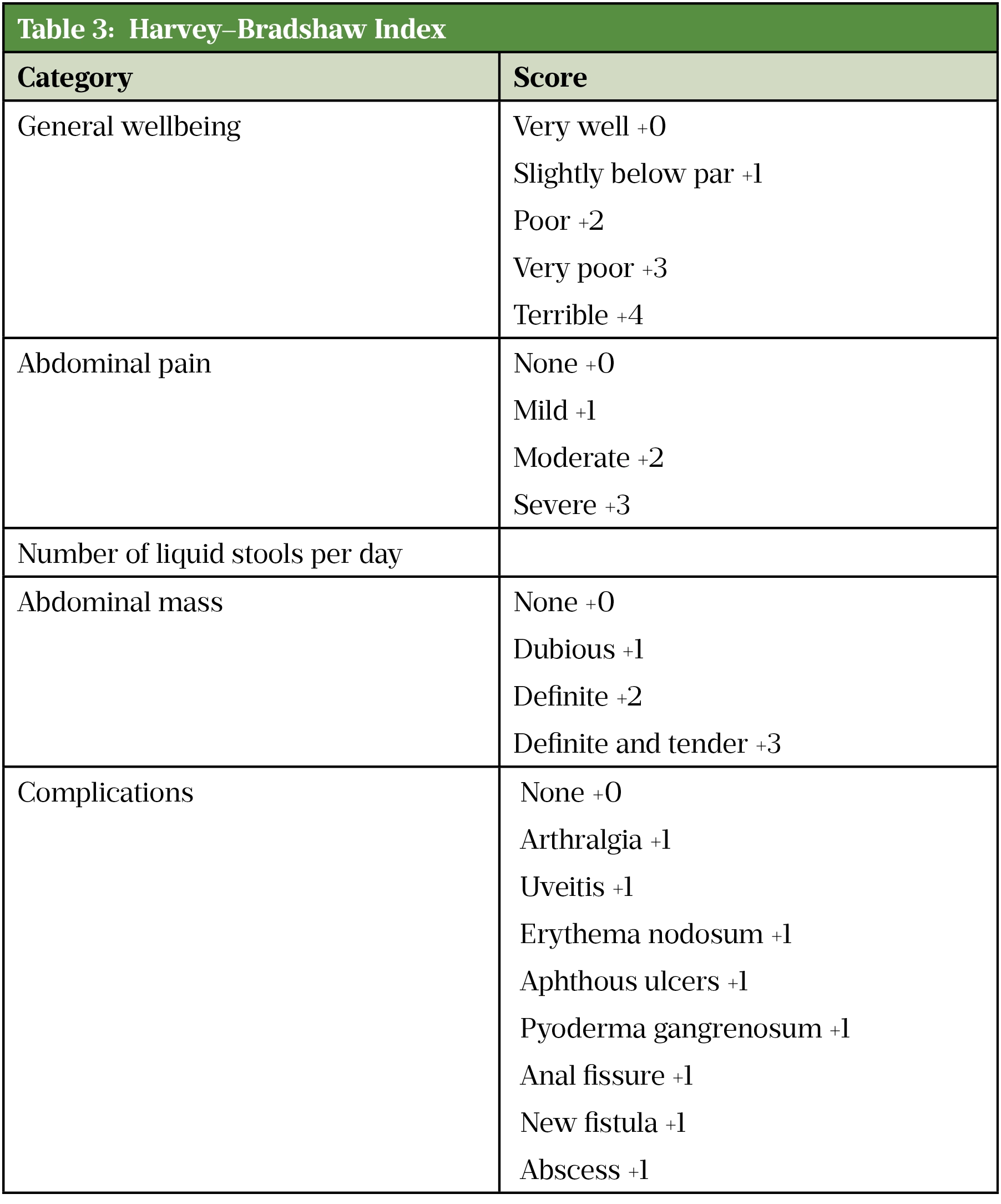
Source: [67]
Ulcerative colitis
Truelove and Witts’ criteria is a useful tool in defining the severity of ulcerative colitis in adults (Table 4)[43]. Acute severe colitis is defined by more than six bloody stools a day plus one or more of the following markers of systemic upset:
- Pulse >90 beats/min;
- Temperature >37.8°C;
- Haemoglobin <10.5g/dL;
- Erythrocyte sedimentation rate (ESR) >30mm/h[43].
This condition is a medical emergency and requires inpatient management for intensive therapy. In such instances, pharmacists should refer patients to their GP or an acute walk-in centre.
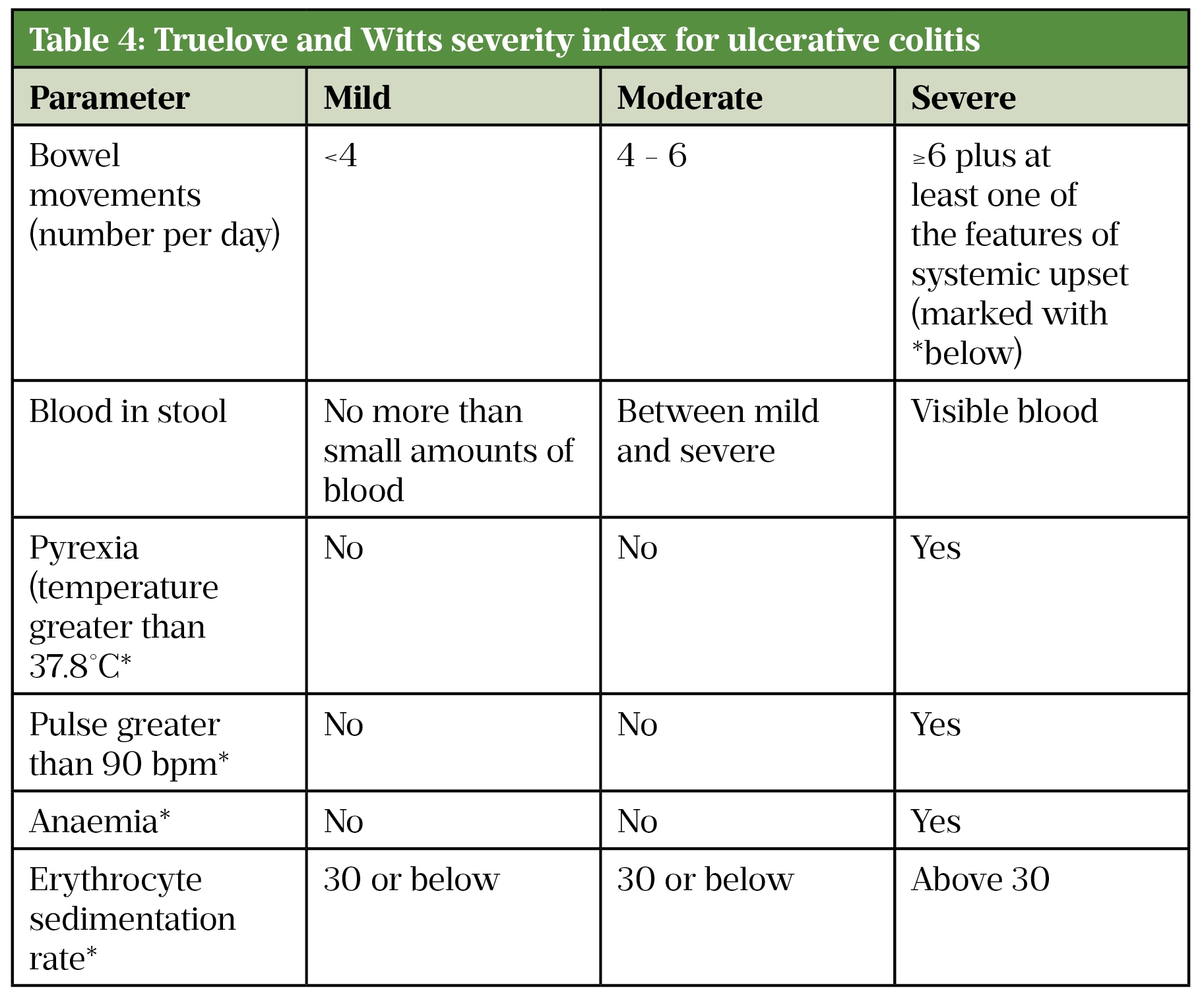
Source: [43]
Patients admitted with acute severe colitis and started on intravenous steroids should be reassessed on day three[68]. A stool frequency of >8 per day on day three, or a stool frequency of 3–8 alongside a raised CRP >45mg/L predicts the need for surgery in 85% of cases and these patients should have therapy intensified (e.g. infliximab, ciclosporin, colectomy)[3,68]. Remission in ulcerative colitis is defined as complete resolution of symptoms with a normal bowel pattern (<3 stools/day), no urgency and no visible bleeding[69].
The Simple Clinical Colitis Activity Index (SCCAI) allows initial evaluation of exacerbations of ulcerative colitis using scores for five clinical criteria, these are ESR, bowel frequency (day and night), urgency of defecation, presence of blood in stool, general well-being and extra-colonic features[70]. A score </= 2 indicates remission.
The Paediatric Ulcerative Colitis Activity Index (PUCAI) is used with children. Scores are based on presence or absence of symptoms (e.g. pain, blood in stools, frequency and consistency of stools and activity levels). A score of 65 or above indicates severe disease[43].
eHealth technology systems (e.g. TrueColours, Constant-Care) utilise patient reported outcomes related to symptoms and quality of life and are increasingly used in chronic illnesses such as IBD[43]. The self-reported scores are remotely accessible to IBD specialist teams to monitor disease activity. They can help manage immediate symptoms as well as identify the longer-term pattern of the illness[71].
Endoscopy
An important diagnostic investigation in IBD is lower gastro-intestinal tract endoscopy (sigmoidoscopy and colonoscopy), which allows direct visualisation of the large bowel/terminal ileum and histopathological assessment of biopsies[72]. Treatment response to biologics is now routinely assessed via mucosal healing seen at colonoscopy[3].
To enable a good view of the mucosa, bowel cleansing agents (e.g. Moviprep) are taken prior to the colonoscopy. Phosphate enemas are usually sufficient for sigmoidoscopy but are contra-indicated in active IBD and gastrointestinal obstruction[73].
The ulcerative colitis endoscopic index of severity (UCEIS) is the only validated endoscopic index in UC[74]. It predicts overall assessment of endoscopic severity of UC. Three descriptors are graded — vascular pattern, bleeding and ulceration to give a score out of 8. A higher score is more predictive of likely colectomy[74]. Remission is defined as score ≤1. UCEIS has reduced variance in endoscopic reporting from 73% to 14%[74].
The CD endoscopic index of severity (CDEIS) or the simple endoscopic score for CD (SES-CD) may be used for endoscopic assessment[62].
Wireless capsule endoscopy allows the small bowel to be viewed and can be useful in patients with non-stricturing CD [75].
Colorectal cancer
Adults with colonic IBD have a higher risk of developing colorectal cancer than the general population[3]. The increased risk is typically associated with the extent, severity and longevity of inflammation, and other factors, such as co-existing primary sclerosing cholangitis (PSC), which is an inflammatory disease of the bile ducts[3,76].
Colonoscopic surveillance in people with IBD can detect abnormal tissue early and potentially prevent progression to colorectal cancer[61]. In the UK, the NHS Bowel Cancer Screening Programme offers screening using faecal immunochemical testing every two years to all men and women aged 60–74 years[77]. Colonoscopic surveillance in the UK should start eight to ten years after diagnosis of IBD or, when relevant, from diagnosis of PSC[3].
Radiology
Radiological imaging for IBD is used in the initial evaluation or diagnosis, preoperative review, to highlight the presence of complications during exacerbations and to evaluate extra-intestinal manifestations[3]. It should be used to support decision making with regards to escalation and de-escalation of treatment.
Radiological examination still plays a key role in IBD affecting the small bowel, although endoscopy has generally replaced conventional radiology of the colon. There are various types of radiological tests and the most appropriate imaging technique is ideally discussed with a radiologist to minimize unnecessary ionising radiation[3].
An abdominal radiograph is essential in acute severe colitis to exclude colonic dilatation and may also help assess disease extent of UC or identify proximal constipation. In CD, abdominal radiography may identify evidence of small bowel dilatation.
Ultrasound and MRI are increasingly used to image the small intestine in CD[3]. Both are safe in pregnancy.
Computed tomography (CT) and magnetic resonance imaging (MRI) are the best radiological methods for locating and defining fistulae and abscesses in CD. MRI has the advantage over CT in that there is no ionizing radiation which is advantageous in a young population where repeat imaging is likely[50].
Radiolabelled leucocyte scans that utilise autologous leucocytes labelled with 99technetium-hexamethylenamine oxime are very rarely used as modern imaging provides superior information about disease severity and complications[50].
Conclusion
In summary, CD and UC are complex conditions whose morbidity burden can have a significant impact on a patient’s life. A combination of symptoms, along with endoscopic, radiological, and histological investigations are used for diagnosis, and to track disease relapse and treatment response. Pharmacists across different sectors will have a role in the care of these patients, this will be covered in the second part of this article, as well as treatment options for the management of both conditions.
Acknowledgements
The author would like to thank Tim Ambrose, consultant gastroenterologist at Oxford University Hospitals NHS Foundation Trust, for his support and guidance.
- 1Cripps S. Inflammatory bowel disease. In: Clinical Pharmacy and Therapeutics. Elsevier 2018. 1112.
- 2Selinger CP, Bannaga A. Inflammatory bowel disease and anxiety: links, risks, and challenges faced. CEG 2015;:111. doi:10.2147/ceg.s57982
- 3Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019;68:s1–106. doi:10.1136/gutjnl-2019-318484
- 4Hendrickson BA, Gokhale R, Cho JH. Clinical Aspects and Pathophysiology of Inflammatory Bowel Disease. Clin Microbiol Rev 2002;15:79–94. doi:10.1128/cmr.15.1.79-94.2002
- 5Vasant DH, Paine PA, Black CJ, et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut 2021;70:1214–40. doi:10.1136/gutjnl-2021-324598
- 6Alatab S, Sepanlou SG, Ikuta K, et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet Gastroenterology & Hepatology 2020;5:17–30. doi:10.1016/s2468-1253(19)30333-4
- 7Crohn’s disease: your guide. Crohn’s & Colitis UK. 2019.https://www.crohnsandcolitis.org.uk/about-crohns-and-colitis/publications/crohns-disease (accessed Aug 2021).
- 8Ulcerative colitis: your guide . Crohn’s & Colitis UK. 2018.www.crohnsandcolitis.org.uk/about-inflammatory-bowel-disease/publications/ulcerative-colitis (accessed Aug 2021).
- 9Pasvol TJ, Horsfall L, Bloom S, et al. Incidence and prevalence of inflammatory bowel disease in UK primary care: a population-based cohort study. BMJ Open 2020;10:e036584. doi:10.1136/bmjopen-2019-036584
- 10Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res 2016;14:111. doi:10.5217/ir.2016.14.2.111
- 11Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2020;18:56–66. doi:10.1038/s41575-020-00360-x
- 12Inflammatory bowel disease. NHS . https://www.nhs.uk/conditions/inflammatory-bowel-disease (accessed Aug 2021).
- 13Crohn’s disease: management. NICE guideline NG129. National Institute for Health and Care Excellence. 2019.https://www.nice.org.uk/guidance/ng129 (accessed Aug 2021).
- 14Silva BC da, Lyra AC, Rocha R, et al. Epidemiology, demographic characteristics and prognostic predictors of ulcerative colitis. WJG 2014;20:9458–67. doi:10.3748/wjg.v20.i28.9458
- 15Nguyen GC, Torres EA, Regueiro M, et al. Inflammatory Bowel Disease Characteristics Among African Americans, Hispanics, and Non-Hispanic Whites: Characterization of a Large North American Cohort. Am J Gastroenterology 2006;101:1012–23. doi:10.1111/j.1572-0241.2006.00504.x
- 16Ran Z, Wu K, Matsuoka K, et al. Asian Organization for Crohn’s and Colitis and Asia Pacific Association of Gastroenterology practice recommendations for medical management and monitoring of inflammatory bowel disease in Asia. Journal of Gastroenterology and Hepatology 2020;36:637–45. doi:10.1111/jgh.15185
- 17Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol 2015;12:205–17. doi:10.1038/nrgastro.2015.34
- 18Kurata JH, Niv Y, Abukasis G. Prevalence of Ulcerative Colitis in the Israeli Kibbutz Population. Journal of Clinical Gastroenterology 1991;13:98–101. doi:10.1097/00004836-199102000-00022
- 19Zhang Y-Z. Inflammatory bowel disease: Pathogenesis. WJG 2014;20:91. doi:10.3748/wjg.v20.i1.91
- 20Feagins LA. Case-control study of factors that trigger inflammatory bowel disease flares. WJG 2014;20:4329. doi:10.3748/wjg.v20.i15.4329
- 21Harris KG, Chang EB. The intestinal microbiota in the pathogenesis of inflammatory bowel diseases: new insights into complex disease. Clinical Science 2018;132:2013–28. doi:10.1042/cs20171110
- 22Loddo I, Romano C. Inflammatory Bowel Disease: Genetics, Epigenetics, and Pathogenesis. Front Immunol 2015;6. doi:10.3389/fimmu.2015.00551
- 23Laharie D, Debeugny S, Peeters M, et al. Inflammatory bowel disease in spouses and their offspring. Gastroenterology 2001;120:816–9. doi:10.1053/gast.2001.22574
- 24Thompson NP, Driscoll R, Pounder RE, et al. Genetics versus environment in inflammatory bowel disease: results of a British twin study. BMJ 1996;312:95–6. doi:10.1136/bmj.312.7023.95
- 25Cleynen I, Boucher G, Jostins L, et al. Inherited determinants of Crohn’s disease and ulcerative colitis phenotypes: a genetic association study. The Lancet 2016;387:156–67. doi:10.1016/s0140-6736(15)00465-1
- 26Bayless T, Tokayer A, Polito J 2nd, et al. Crohn’s disease: Concordance for site and clinical type in affected family members–potential hereditary influences. Gastroenterology 1996;111:573–9. doi:10.1053/gast.1996.v111.pm8780559
- 27Santos MPC. ‘ Familial and ethnic risk in inflammatory bowel disease’. aog Published Online First: 2017. doi:10.20524/aog.2017.0208
- 28Ye BD, McGovern DPB. Genetic variation in IBD: progress, clues to pathogenesis and possible clinical utility. Expert Review of Clinical Immunology 2016;12:1091–107. doi:10.1080/1744666x.2016.1184972
- 29Duerr RH, Taylor KD, Brant SR, et al. A Genome-Wide Association Study Identifies IL23R as an Inflammatory Bowel Disease Gene. Science 2006;314:1461–3. doi:10.1126/science.1135245
- 30Aggeletopoulou I, Assimakopoulos SF, Konstantakis C, et al. Interleukin 12/interleukin 23 pathway: Biological basis and therapeutic effect in patients with Crohn’s disease. WJG 2018;24:4093–103. doi:10.3748/wjg.v24.i36.4093
- 31Reddavide R, Rotolo O, Caruso MG, et al. The role of diet in the prevention and treatment of Inflammatory Bowel Diseases. Acta Bio Medica Atenei Parmensis 2018;89:60–75. doi:10.23750/abm.v89i9-S.7952
- 32Desreumaux P, Ernst O, Geboes K, et al. Inflammatory alterations in mesenteric adipose tissue in Crohn’s disease. Gastroenterology 1999;117:73–81. doi:10.1016/s0016-5085(99)70552-4
- 33Xu L, Lochhead P, Ko Y, et al. Systematic review with meta-analysis: breastfeeding and the risk of Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther 2017;46:780–9. doi:10.1111/apt.14291
- 34Mahid SS, Minor KS, Soto RE, et al. Smoking and Inflammatory Bowel Disease: A Meta-analysis. Mayo Clinic Proceedings 2006;81:1462–71. doi:10.4065/81.11.1462
- 35Ledder O. Antibiotics in inflammatory bowel diseases: do we know what we’re doing? Transl Pediatr 2019;8:42–55. doi:10.21037/tp.2018.11.02
- 36Ungaro R, Bernstein CN, Gearry R, et al. Antibiotics Associated With Increased Risk of New-Onset Crohn’s Disease But Not Ulcerative Colitis: A Meta-Analysis. American Journal of Gastroenterology 2014;109:1728–38. doi:10.1038/ajg.2014.246
- 37Davis RL. Measles-Mumps-Rubella and Other Measles-Containing Vaccines Do Not Increase the Risk for Inflammatory Bowel Disease<subtitle>A Case-Control Study From the Vaccine Safety Datalink Project</subtitle>. Arch Pediatr Adolesc Med 2001;155:354. doi:10.1001/archpedi.155.3.354
- 38Ananthakrishnan A. Environmental risk factors for inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2013;9:367–74.https://www.ncbi.nlm.nih.gov/pubmed/23935543
- 39Kaplan GG, Jackson T, Sands BE, et al. The Risk of Developing Crohn’s Disease After an Appendectomy: A Meta-Analysis. The American Journal of Gastroenterology 2008;103:2925–31. doi:10.1111/j.1572-0241.2008.02118.x
- 40Sun Y, Li L, Xie R, et al. Stress Triggers Flare of Inflammatory Bowel Disease in Children and Adults. Front Pediatr 2019;7. doi:10.3389/fped.2019.00432
- 41Mawdsley JE. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut 2005;54:1481–91. doi:10.1136/gut.2005.064261
- 42Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. The Lancet 2017;389:1756–70. doi:10.1016/s0140-6736(16)32126-2
- 43Ulcerative colitis: management. NICE guideline 130. National Institute for Health and Care Excellence. 2019.www.nice.org.uk/ng130 (accessed Aug 2021).
- 44McDowell C, Farooq U, Haseeb M. Inflammatory Bowel Disease. StatPearls Publishing. 2021.https://www.ncbi.nlm.nih.gov/books/NBK470312/ (accessed Aug 2021).
- 45Rendi M. Crohn Disease Pathology. Medscape. 2017.https://emedicine.medscape.com/article/1986158-overview (accessed Aug 2021).
- 46Axelrad JE, Lichtiger S, Yajnik V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. WJG 2016;22:4794. doi:10.3748/wjg.v22.i20.4794
- 47Townsend T, Campbell F, O’Toole P, et al. Microscopic colitis: diagnosis and management. Frontline Gastroenterol 2019;10:388–93. doi:10.1136/flgastro-2018-101040
- 48Kurien M, Raju S. Management of difficult microscopic colitis after failure of budesonide therapy. British Society of Gastroenterology. 2019.https://www.bsg.org.uk/clinical-articles-list/management-of-difficult-microscopic-colitis-after-failure-of-budesonide-therapy/ (accessed Aug 2021).
- 49Editorial team. Diversion Colitis. Inflammatory Bowel Disease. 2018.https://inflammatoryboweldisease.net/types-of-ibd/diversion-colitis (accessed Aug 2021).
- 50Farooq PD, Urrunaga NH, Tang DM, et al. Pseudomembranous colitis. Disease-a-Month 2015;61:181–206. doi:10.1016/j.disamonth.2015.01.006
- 51Magro F, Gionchetti P, Eliakim R, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. Journal of Crohn’s and Colitis 2017;11:649–70. doi:10.1093/ecco-jcc/jjx008
- 52Bemelman WA, Warusavitarne J, Sampietro GM, et al. ECCO-ESCP Consensus on Surgery for Crohn’s Disease. Journal of Crohn’s and Colitis Published Online First: 11 May 2017. doi:10.1093/ecco-jcc/jjx061
- 53Fistulas. Crohn’s and Colitis UK. 2020.https://www.crohnsandcolitis.org.uk/about-crohns-and-colitis/publications/fistulas (accessed Aug 2021).
- 54Marzo M. Management of perianal fistulas in Crohn’s disease: An up-to-date review. WJG 2015;21:1394. doi:10.3748/wjg.v21.i5.1394
- 55Cripps S. Inflammatory bowel disease: NICE updates advice on remission. Guidelines in Practice. 2019.https://www.guidelinesinpractice.co.uk/gastrointestinal/inflammatory-bowel-disease-nice-updates-advice-on-remission/454783.article (accessed Aug 2021).
- 56Solem CA, Loftus EV, Tremaine WJ, et al. Venous Thromboembolism in Inflammatory Bowel Disease. Am J Gastroenterol 2004;99:97–101. doi:10.1046/j.1572-0241.2003.04026.x
- 57Autenrieth DM, Baumgart DC. Toxic megacolon. Inflammatory Bowel Diseases 2012;18:584–91. doi:10.1002/ibd.21847
- 58Complications ulcerative colitis. NHS. 2019.https://www.nhs.uk/conditions/ulcerative-colitis/complications/ (accessed Aug 2021).
- 59Short bowel syndrome. National Institute of Diabetes and Digestive and Kidney Diseases. 2015.https://www.niddk.nih.gov/health-information/digestive-diseases/short-bowel-syndrome (accessed Aug 2021).
- 60Annese V, Beaugerie L, Egan L, et al. European Evidence-based Consensus: Inflammatory Bowel Disease and Malignancies. Journal of Crohn’s and Colitis 2015;9:945–65. doi:10.1093/ecco-jcc/jjv141
- 61Colorectal cancer prevention: colonoscopic surveillance in adults with ulcerative colits, crohn’s diease or adenomas. NICE Clinical Guideline 118. National Institute for Health and Care Excellence. 2011.www.nice.org.uk/cg118 (accessed Aug 2021).
- 62Walsh AJ, Bryant RV, Travis SPL. Current best practice for disease activity assessment in IBD. Nat Rev Gastroenterol Hepatol 2016;13:567–79. doi:10.1038/nrgastro.2016.128
- 63Shivashankar R, Lichtenstein GR. Mimics of Inflammatory Bowel Disease. Inflammatory Bowel Diseases 2018;24:2315–21. doi:10.1093/ibd/izy168
- 64Justice S, James T, Maunsell-Browne Z. Biochemistry Reference Ranges Document. Oxford University Hospitals NHS Foundation Trust, Department of Clinical Biochemistry. 2019.https://www.ouh.nhs.uk/biochemistry/tests/documents/biochemistry-reference-ranges.pdf (accessed Aug 2021).
- 65Crohn’s Disease Activity Index (CDAI). MD+CALC. https://www.mdcalc.com/crohns-disease-activity-index-cdai (accessed Aug 2021).
- 66Harvey-Bradshaw Index (HBI) for Crohn’s Disease. MD+CALC. https://www.mdcalc.com/harvey-bradshaw-index-hbi-crohns-disease (accessed Aug 2021).
- 67Harvey RF, Bradshaw JM. A SIMPLE INDEX OF CROHN’S-DISEASE ACTIVITY. The Lancet 1980;315:514. doi:10.1016/s0140-6736(80)92767-1
- 68Travis SP, Farrant JM, Ricketts C, et al. Predicting outcome in severe ulcerative colitis. Gut 1996;38:905–10. doi:10.1136/gut.38.6.905
- 69Travis SPL, Higgins PDR, Orchard T, et al. Review article: defining remission in ulcerative colitis. Alimentary Pharmacology & Therapeutics 2011;34:113–24. doi:10.1111/j.1365-2036.2011.04701.x
- 70Walmsley RS, Ayres RCS, Pounder RE, et al. A simple clinical colitis activity index. Gut 1998;43:29–32. doi:10.1136/gut.43.1.29
- 71Walsh A, Travis S. What’s app? Electronic health technology in inflammatory bowel disease. Intest Res 2018;16:366. doi:10.5217/ir.2018.16.3.366
- 72Spiceland CM, Lodhia N. Endoscopy in inflammatory bowel disease: Role in diagnosis, management, and treatment. WJG 2018;24:4014–20. doi:10.3748/wjg.v24.i35.4014
- 73Sodium acid phosphate with sodium phosphate. British National Formulary. 2021.https://bnf.nice.org.uk/drug/sodium-acid-phosphate-with-sodium-phosphate.html#contraIndications (accessed Aug 2021).
- 74Travis SPL, Schnell D, Krzeski P, et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut 2011;61:535–42. doi:10.1136/gutjnl-2011-300486
- 75Mitselos IV, Christodoulou DK, Katsanos KH, et al. Role of wireless capsule endoscopy in the follow-up of inflammatory bowel disease. WJGE 2015;7:643. doi:10.4253/wjge.v7.i6.643
- 76Kim ER. Colorectal cancer in inflammatory bowel disease: The risk, pathogenesis, prevention and diagnosis. WJG 2014;20:9872. doi:10.3748/wjg.v20.i29.9872
- 77Bowel cancer screening: programme overview. Gov.uk. 2021.https://www.gov.uk/guidance/bowel-cancer-screening-programme-overview (accessed Aug 2021).