Shutterstock.com
After reading this article, you should be able to:
- Understand the insulin regimens used in the management of type 2 diabetes;
- Know the insulins available in the UK according to their time-action profile;
- Know how to support people with type 2 diabetes who are on insulin;
- Understand the glucagon-like peptide-1 receptor agonists/insulin combination products available in the UK.
The use of insulin in type 1 diabetes mellitus is life-saving owing to the absolute insulin deficiency resulting from autoimmune destruction of the beta cells in the pancreas[1]. Type 2 diabetes mellitus (T2DM) is characterised as a progressive disease owing to a combination of the development of insulin resistance and continuing decline of beta cell function[2]. Although glycaemic control is usually achieved by other glucose-lowering therapies — such as metformin, sulfonylureas, pioglitazone, dipeptidyl peptidase 4 (DPP4) inhibitors, sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagon-like peptide-1 receptor agonists (GLP-1 RA) — some people with T2DM may need insulin to achieve optimal glycaemic control[3].
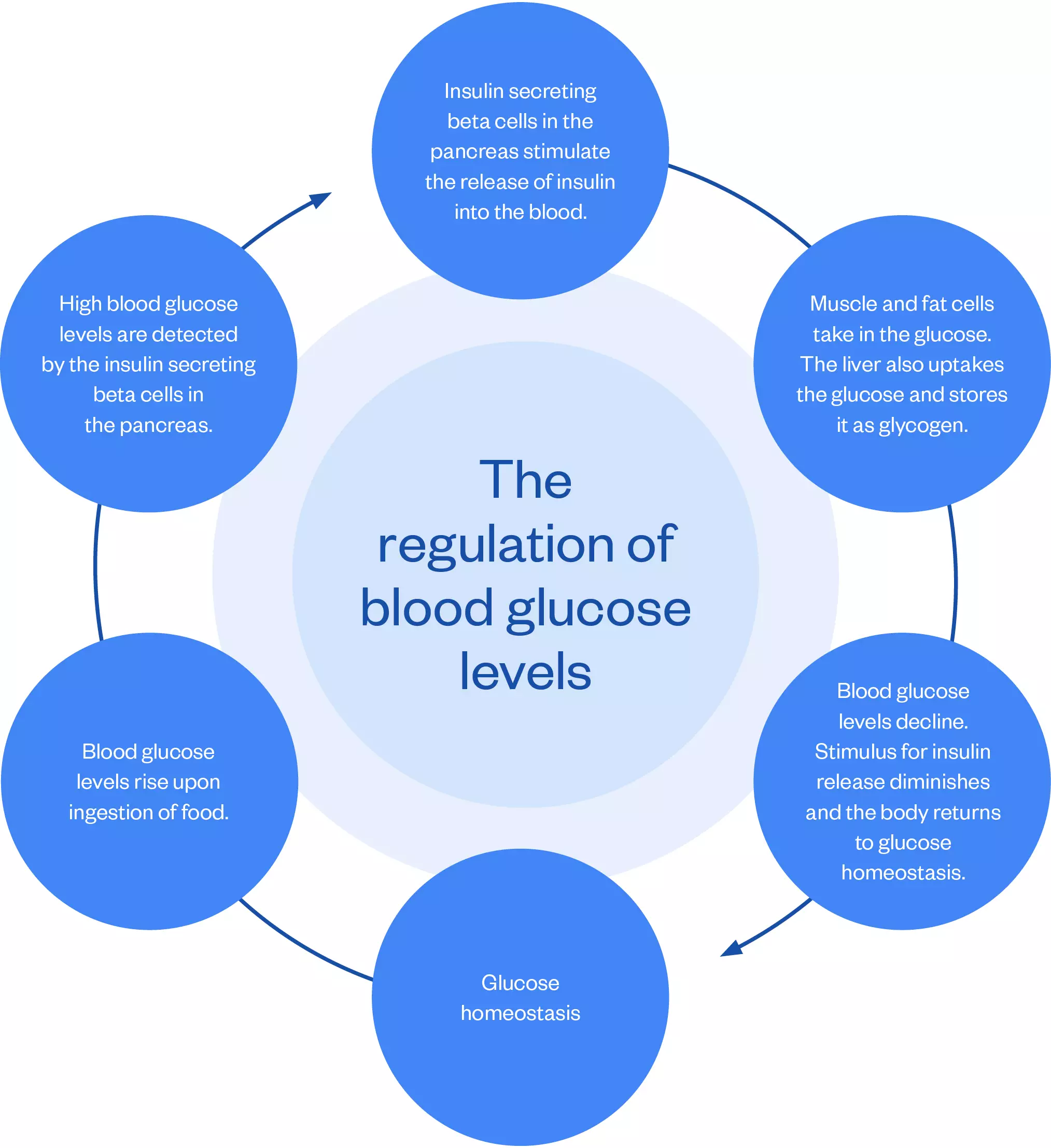
Insulin is a polypeptide hormone secreted by the beta cells of the islets of Langerhans in the pancreas[4]. The main role of insulin is to lower blood glucose levels following the ingestion of food, as depicted in the figure below.
There has been a considerable increase in the number of people with T2DM taking insulin in the UK; with around one in four people receiving this medication[5,6]. This increase may be owing to individuals experiencing a longer duration of T2DM because they are diagnosed earlier and living longer[5].
Pharmacists working in all sectors are increasingly likely to encounter people with T2DM who take insulin and therefore must have an understanding of the types of insulin available, insulin regimens and relevant counselling points related to insulin management so they can advise people with T2DM who are on insulin competently and confidently. This article focuses on the use of insulin in the management of T2DM; additional information on management can be found in ‘Diagnosis and management of type 2 diabetes mellitus‘.
Insulin and time-action profiles
There are three types of insulin available in the UK: animal insulin, human insulin and human insulin analogue[4]. Animal insulin is no longer recommended for use to initiate insulin therapy because it has been replaced by human insulin and human analogue insulin[7,8]. Insulins are categorised according to their time-action profiles (see Table 1):
Short-acting, rapid-acting and ultra-rapid acting insulins mimic the physiological secretion of insulin that occurs in response to the sharp rise of glucose following the ingestion of food or drink.
Intermediate-acting, long-acting and ultra-long acting insulins mimic the effect of basal insulin that is continuously released from the liver and is relatively constant but declines slightly during the night and peaks just before dawn[4].
In the UK, there are four biosimilar insulins available. Biosimilar insulins are produced when a patent expires on an insulin, allowing manufacturers to produce copies of the original insulin molecule. There are several benefits from using biosimilar insulins; for example, they are less expensive than the parent insulin, and large-scale randomised control trials needed for the parent insulin are not required for the biosimilar insulin — instead, the biosimilar insulin needs to prove bioequivalence to the parent insulin[9]. See Table 1 for the types of insulin available in the UK, categorised according to their time-action profiles[10–12].
Individualised HbA1c treatment targets
Results from the ‘UK Prospective Diabetes Study’ (UKPDS) show that intensive glycaemic control is associated with a reduced risk of long-term diabetes-related complications, including microvascular complications (e.g. retinopathy, nephropathy and neuropathy), macrovascular complications (e.g. cardiovascular disease, cerebrovascular disease and peripheral arterial disease) and metabolic complications (e.g. dyslipidaemia and diabetic ketoacidosis)[3,5].
Although the National Institute for Health and Care Excellence (NICE) and Scottish Intercollegiate Guidelines Network (SIGN) recommend a HbA1c treatment target of ≤53mmol/mol (or a HbA1c target of ≤48mmol/mol at diagnosis) and ≤48mmol/mol, respectively, it is important to note that HbA1c treatment targets should be individualised and agreed with the person living with diabetes[13,14]. This can depend on whether the person has a predictable or erratic lifestyle, any existing comorbidities (e.g. atherosclerotic cardiovascular disease, chronic kidney disease or heart failure), their age, weight, risk of hypoglycaemia, life expectancy and motivation on managing their condition[15].
HbA1c, which depicts the average blood glucose levels for the past two to three months, should be checked regularly at three-monthly intervals by the person’s diabetes healthcare team (GP, pharmacist or practice nurse) to ensure the individual has achieved their HbA1c treatment target, and then every six months once the target has been achieved[15,16].
Both NICE and SIGN advocate the use of insulin as third- or fourth-line therapy when the person’s HbA1c is above their individualised target, despite maximal or near maximal doses of glucose-lowering medicines[13,14]. Consult the British National Formulary for further information on maximal doses of glucose-lowering drugs.
Insulin initiation should also be considered in the following situations:
- Worsening symptoms of hyperglycaemia, such as polyuria, polydipsia, tiredness, weight loss and blurred vision[17];
- Intercurrent illness or patient commenced on steroid therapy[7].
Insulin regimens in the management of type 2 diabetes mellitus
The 4T study evaluated the insulin regimens used in the management of T2DM that are covered in this article and concluded that, although the twice-daily biphasic insulin regimen and thrice-daily bolus (meal time) regimen reduced HbA1c more than the once-daily basal regimen, there was an increased risk of weight gain and hypoglycaemia, which are known adverse effects of insulin[18,19]. Therefore, the choice of insulin regimen should be individualised — following a discussion with the individual — and will depend on their preference for injection frequency, self-monitoring of blood glucose, regular or variable eating pattern, as well their capability of understanding the regimen and their access to family support[20]. This case-based learning article looks at how best to approach insulin intensification in a way that is patient-centred.
Once-daily basal insulin
Once-daily basal insulin is the simplest regimen[21]. When compared to intermediate-acting insulin or neutral protamine hagedorn (NPH) insulin — which is an isophane suspension of human insulin — basal analogues have been shown to have fewer episodes of hypoglycaemia, including nocturnal hypoglycaemia, but no difference in severe hypoglycaemia[14]. However, there is minimal difference in HbA1c reduction between the two insulins. Therefore, both NICE and SIGN recommend using NPH insulin as first line and to consider basal analogues as an alternative to NPH insulin if:
- The person needs assistance from a carer or healthcare professional to inject insulin and for whom switching to one of the long-acting insulin analogues would reduce the number of daily injections;
- The person’s lifestyle is restricted by recurrent symptomatic hypoglycaemic episodes;
- The person would otherwise need twice-daily NPH insulin in combination with oral blood glucose-lowering drugs[13,14].
Further indications for considering basal analogues suggested by SIGN include:
- Those who suffer from recurrent episodes of hypoglycaemia, particularly at night:
- Those for whom hypoglycaemia could cause increased risk to themselves or others; for example, occupational driving, working with heavy machinery, working at heights (if this cannot be avoided), or caring for young or otherwise vulnerable individuals[14].
NPH insulin is usually injected once daily before bed or in the evening[22]. Since the time-action profile of NPH insulin does not cover 24 hours, some people may need twice-daily dosing, morning and evening or bedtime, if their fasting blood glucose targets have not been achieved by once-daily dosing of NPH insulin[23]. Basal analogues are injected once daily, with Levemir (insulin detemir; Novo Nordisk) being the exception as it can be injected once or twice daily[24].
Despite NPH insulin having a slight peak (the time during which insulin is at maximum strength in lowering blood glucose levels), it has a reduced risk of both hypoglycaemia and weight gain, making this regimen suitable for those who are reluctant to start insulin treatment, are overweight, or for intensifying regimens in older people who would otherwise have an increased risk of hypoglycaemia[20,23,25].
It is important to note that basal insulin does not control post-prandial (i.e. after eating) rises and therefore may not provide optimal glycaemic control. If there are post-prandial rises, a bolus dose may be added before the meal with the highest post-prandial excursion (this regimen is called basal-plus)[26,27]. Additionally, NPH insulin must be re-suspended and thus requires adequate mixing before each use[22].
Consider changing the individual’s insulin regimen to a twice-daily biphasic or a basal/bolus one, depending on the factors that influence regimen choice outlined previously, if their HbA1c or fasting blood glucose or both are sub optimal; when fasting glucose levels are at target, but postprandial glucose levels remain high despite maximum tolerated glucose-lowering medicines; or in recurring and/or unresolving hypoglycaemia and/or nocturnal hypoglycaemia[24].
Further guidance on how to switch the individual to another insulin regimen can be found here.
Twice-daily regimen
The twice-daily regimen should be considered in those with an HbA1c ≥75mmol/mol[13], or as treatment intensification for those who do not achieve HbA1c targets despite basal insulin being an add-on to other glucose-lowering therapies[24].
NPH insulin in combination with short-acting insulin administered either separately or as biphasic human insulin preparation is first line for this regimen because there is little difference in HbA1c reduction and hypoglycaemia rates when compared with biphasic preparations that include rapid-acting analogues[13,14,28]. However, biphasic preparations that include rapid-acting analogue insulin should be considered if:
- The person prefers injecting insulin immediately before a meal;
- Hypoglycaemia is a problem;
- Blood glucose levels rise markedly after meals;
- The person does not snack between meals[13,14].
Biphasic insulin preparations are usually injected twice daily — before breakfast and before the evening meal. Human biphasic preparations are injected 20–30 minutes before meals, whereas biphasic analogue preparations can be injected immediately before meals. In some cases of sub-optimal control, a third dose can be injected before lunch[29]. There is lack of flexibility in dose titration of the fixed ratio of long-acting and short-acting components[22].
This regimen is simple and easy to initiate and is effective in addressing both fasting and prandial glycaemia. However, there may be a higher risk of hypoglycaemia and weight gain[24]. Weight gain can be minimised by advising the individual to follow a healthy diet and exercise regularly[30]. The risk of hypoglycaemia can be mitigated by advising the individual to eat regular meals, reduce the dose of insulin before planned exercise, ensure they are monitoring their blood glucose levels regularly, and titrating or de-titrating their insulin doses according to their blood glucose levels (for further information, see Table 2)[24,31].
Consider changing the individual’s insulin regimen to basal/bolus in the presence of suboptimal HbA1c or fasting plasma glucose or both; if pre-evening meal blood glucose levels are high and further increase in morning insulin dose results in mid-morning hypoglycaemia, or simply owing to patient preference or perception of lack of flexibility with regimen[24].
Basal bolus
This regimen involves multiple injections; short- or rapid-acting insulin is injected before meals and basal insulin is injected once or twice daily, depending on the insulin. This regimen mimics normal physiology and gives more flexibility to those who have an erratic lifestyle, irregular eating habits or are shift workers[32]. This regimen is efficacious and provides a higher chance of achieving HbA1c treatment target, but this depends on the motivation of the individual[24].
As previously mentioned, NPH insulin is first choice for basal insulin and short-acting insulin is also preferred as first line over rapid acting analogues[13,14].
As this regimen carries the highest risk of hypoglycaemia, the individual will have to monitor their blood glucose levels regularly by either finger pricking, using a flash glucose monitor or a continuous glucose monitor[33].
The insulin regimen can be changed if the individual is unwilling to continue taking multiple daily injections in order to improve medication adherence and quality of life, to either a once-daily fixed-combination injection of basal insulin with a GLP-1RA or a sodium glucose co-transporter-2 inhibitor (SGLT2i) added to basal insulin, to produce similar glucose control as the basal bolus regimen but with the added benefit of fewer insulin doses, fewer injections daily and reduced risk of hypoglycaemia[34,35].
Insulin starting doses, dose adjustment and monitoring
Table 2 depicts the starting doses for the different insulin regimens, monitoring requirements and when and how to titrate or de-titrate insulin as per blood glucose readings[26].
Counselling points to communicate at point of initiating insulin
The following points should be discussed with the individual at the point of initiating insulin. It is important to remember that education on using insulin is a continual process and should be given as and when necessary:
- Hypoglycaemia: determine awareness and understanding of recognising, managing and identifying the causes of hypoglycaemia. Advice should be given on minimising hypoglycaemia risk;
- Insulin administration technique: verify understanding of insulin administration, storage, injection technique and rotation of injection sites, including discarding of pen needles after each use;
- Acute changes in blood glucose levels: managing insulin treatment during periods of intercurrent illness or during acute changes in blood glucose levels;
- Titration of insulin: check understanding of titration/de-titration of insulin dose according to blood glucose levels and awareness of individualised blood glucose/HbA1c targets;
- Blood glucose monitoring: advise when to monitor blood glucose levels according to person’s insulin regimen;
- Lifestyle aspects of insulin treatment: confirm understanding the effect of diet, alcohol consumption, travelling to warmer climates and exercise on blood glucose levels. Check awareness of driving rules, including when to monitor blood glucose levels, informing the Driver and Vehicle Licensing Agency and managing hypoglycaemia while driving. Check if able to manage their insulin at work safely;
- Cultural aspects of insulin treatment: establish awareness of managing insulin doses when deciding to fast. More detailed considerations can be found in ‘Case-based learning: insulin intensification in a Hindu patient with type 2 diabetes mellitus’ and ‘How pharmacists can support and advise patients during Ramadan’[4,7].
Continuing other glucose-lowering medicines
The person should continue their prescribed glucose lowering drugs alongside insulin, unless there has not been a reduction in HbA1c by at least 5.5mmol/mol in the first six months of starting treatment[14]. Additional consideration is needed if the person is on the following glucose-lowering drugs:
- Metformin should be continued if tolerated (i.e. minimal gastrointestinal adverse effects), or if the estimated glomerular filtration rate (eGFR) permits. Stop metformin if eGFR ≤30mL/minute/1.73m2 or reduce the dose of metformin to 500mg twice daily if eGFR ≤45ml/minute/1.73m2[17];
- Sulfonylurea dose should be reduced by half or consider stopping to reduce risk of hypoglycaemia[36];
- When using SGLT2i/GLP-1RAs, any benefits, such as renal protection, cardiovascular disease benefit or weight management should be confirmed and the safety reviewed to evaluate continued and/or impact of discontinuation of the treatment[36].
Fixed insulin and GLP-1 RA combinations
GLP-1 RAs are available in combination with a basal insulin in a fixed dose ratio for a once-daily injection at any time of the day, at the same time each day (see Table 3 for information on components)[37,38]. The basal insulin reduces fasting glucose levels and the GLP-1 RA reduces postprandial glucose levels and mitigates insulin-induced weight gain and a lower rate of hypoglycaemia[39]. The combination product is more effective in lowering HbA1c and more patients achieve an HbA1c ≤ 53mmol/mol than using the components individually[40]. Therefore, the combination product should be considered for those individuals who require insulin, but where any weight gain induced by insulin is detrimental. Additional information on the initiation of GLP-1 RAs and their place in the management can be found in ‘Glucagon-like peptide-1 analogues in adults with type 2 diabetes mellitus for glycaemic control’.
Best practice
- Familiarise yourself with local formulary insulin;
- Remember that the insulin regimen and glucose targets/HbA1c should reflect the needs of the individual and be chosen to match the person’s life;
- Consider the level of support the individual starting insulin may require in terms of regular appointments either via phone call or face to face from a healthcare professional to advise on insulin dosage adjustment;
- Use checklist at initiation to confirm what has been covered. A template checklist can be found here.
- 1Diabetes – type 1. National Institute for Health and Care Excellence. 2022.https://cks.nice.org.uk/topics/diabetes-type-1 (accessed Dec 2022).
- 2Galicia-Garcia U, Benito-Vicente A, Jebari S, et al. Pathophysiology of Type 2 Diabetes Mellitus. IJMS. 2020;21:6275. doi:10.3390/ijms21176275
- 3King P, Peacock I, Donnelly R. The UK Prospective Diabetes Study (UKPDS): clinical and therapeutic implications for type 2 diabetes. British Journal of Clinical Pharmacology. 1999;48:643–8. doi:10.1046/j.1365-2125.1999.00092.x
- 4Insulin therapy in type 2 diabetes. National Institute for Health and Care Excellence. 2021.www.cks.nice.org.uk/topics/insulin-therapy-in-type-2-diabetes (accessed Dec 2022).
- 5Holden SE, Barnett AH, Peters JR, et al. The incidence of type 2 diabetes in the United Kingdom from 1991 to 2010. Diabetes Obes Metab. 2013;15:844–52. doi:10.1111/dom.12123
- 6Insulin and type 2 diabetes. Diabetes UK. 2022.https://www.diabetes.org.uk/guide-to-diabetes/managing-your-diabetes/treating-your-diabetes/insulin/type-2-diabetes (accessed Dec 2022).
- 7Diabetes: Insulin Initiation and Adjustment, Patients with Type 2 Primary Care: Guidance for Diabetes Specialist Nurses. NHS Greater Glasgow and Clyde. 2019.https://clinicalguidelines.nhsggc.org.uk/media/2070/diabetes-insulin-initiation-and-adjustment-t2-guidance-for-diabetes-specialist-nurses.pdf (accessed Dec 2022).
- 8Insulin. Norfolk and Norwich University Hospitals. 2022.https://www.nnuh.nhs.uk/departments/diabetes-and-endocrinology/diabetes/living-with-diabetes/medications/insulin/ (accessed Dec 2022).
- 9Morris D. Biosimilar insulins: An in-depth guide. Journal of Diabetes Nursing. 2022.www.diabetesonthenet.com/wp-content/uploads/228.-Morris-Biosimilars.pdf (accessed Dec 2022).
- 10Insulin Comparison and Identification Guide. NHS Pan Merseyside. 2019.https://www.panmerseyapc.nhs.uk/document-store/insulin-comparison-and-identification-guide/ (accessed Dec 2022).
- 11Insulin Guidelines for Adults and Children with Type 1 or Type 2 Diabetes Mellitus Diabetes Medicines Management Advisory Group (DMMAG). Birmingham, Sandwell, Solihull and Environs (BSSE) APC. 2022.http://www.birminghamandsurroundsformulary.nhs.uk/docs/acg/Insulin%20Guidelines%20for%20Adults%20and%20Children%20with%20Type%201%20and%20Type%202%20Diabetes%20Mellitus%20V%201.0%20%20February%202021.pdf (accessed Dec 2022).
- 12Typical Insulin Profiles. University Hospitals of Leicester. 2017.https://www.diabetes.org.uk/resources-s3/2017-10/University%2520Hospitals%2520of%2520Leicester%2520-%2520Insulin%2520Profiles.pdf (accessed Dec 2022).
- 13Type 2 diabetes in adults: management . National Institute for Health and Care Excellence. 2022.https://www.nice.org.uk/guidance/ng28/resources/type-2-diabetes-in-adults-management-pdf-1837338615493 (accessed Dec 2022).
- 14Management of diabetes 116 A national clinical guideline. Scottish Intercollegiate Network Guideline. 2017.https://www.sign.ac.uk/assets/sign116.pdf (accessed Dec 2022).
- 15Davies MJ, Aroda VR, Collins BS, et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45:2753–86. doi:10.2337/dci22-0034
- 16Meet your healthcare team. Diabetes UK. 2017.https://www.diabetes.org.uk/guide-to-diabetes/managing-your-diabetes/interactions-with-healthcare-professionals (accessed Dec 2022).
- 17What is type 2 diabetes? NHS. https://www.nhs.uk/conditions/type-2-diabetes (accessed Dec 2022).
- 18Holman RR, Farmer AJ, Davies MJ, et al. Three-Year Efficacy of Complex Insulin Regimens in Type 2 Diabetes. N Engl J Med. 2009;361:1736–47. doi:10.1056/nejmoa0905479
- 19Type 2 diabetes. National Institute for Health and Care Excellence. https://bnf.nice.org.uk/treatment-summaries/type-2-diabetes (accessed Dec 2022).
- 20Barnett A, Begg A, Dyson P, et al. Insulin for type 2 diabetes: choosing a second-line insulin regimen. International Journal of Clinical Practice. 2008;62:1647–53. doi:10.1111/j.1742-1241.2008.01909.x
- 21Gadsby R. Insulin initiation in primary care. Diabetes and Primary Care. 2007.https://www.woundsme.com/uploads/resources/dotn/_master/519/files/pdf/dpc9-6-338-42.pdf (accessed Dec 2022).
- 22Bazzano L, Lee L, Shi L, et al. Safety and efficacy of glargine compared with NPH insulin for the treatment of Type 2 diabetes: a meta-analysis of randomized controlled trials. Diabet Med 2008;25:924–32. doi:10.1111/j.1464-5491.2008.02517.x
- 23Sharma A, Saleem F. NPH Insulin. National Library of Medicine. 2022.https://www.ncbi.nlm.nih.gov/books/NBK549860 (accessed Dec 2022).
- 24Gururaj S, Crasto W, Jarvis J, et al. New insulins and newer insulin regimens: a review of their role in improving glycaemic control in patients with diabetes. Postgrad Med J 2016;92:152–64. doi:10.1136/postgradmedj-2015-133716
- 25Insulin Basics . American Diabetes Association. 2022.https://diabetes.org/healthy-living/medication-treatments/insulin-other-injectables/insulin-basics#:~:text=Peak%20time%20is%20the%20time (accessed Dec 2022).
- 26Insulin Prescribing Guidance: Type 2 Diabetes. NHS Fife. 2021.www.fifeadtc.scot.nhs.uk/media/6978/insulin-guidance-document-v040_mcn_msdtc-approved_27042021.pdf (accessed Dec 2022).
- 27Lankisch M, Del P, Dain M, et al. Use of a basal-plus insulin regimen in persons with type 2 diabetes stratified by age and body mass index: A pooled analysis of four clinical trials. Prim Care Diabetes 2016;10:51–9. doi:10.1016/j.pcd.2015.05.003
- 28Garber A, Ligthelm R, Christiansen J, et al. Premixed insulin treatment for type 2 diabetes: analogue or human? Diabetes Obes Metab 2007;9:630–9. doi:10.1111/j.1463-1326.2006.00654.x
- 29Elizarova S, Galstyan G, Wolffenbuttel B. Role of premixed insulin analogues in the treatment of patients with type 2 diabetes mellitus: a narrative review. J Diabetes 2014;6:100–10. doi:10.1111/1753-0407.12096
- 30Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes–causes, effects and coping strategies. Diabetes Obes Metab 2007;9:799–812. doi:10.1111/j.1463-1326.2006.00686.x
- 31Zaharieva D, Riddell M. Prevention of exercise-associated dysglycemia: a case study-based approach. Diabetes Spectr 2015;28:55–62. doi:10.2337/diaspect.28.1.55
- 32Rosenstock J. Insulin therapy: optimizing control in type 1 and type 2 diabetes. Clin Cornerstone 2001;4:50–64. doi:10.1016/s1098-3597(01)90029-8
- 33Diabetes and Checking Your Blood Sugars. Diabetes UK. 2022.https://www.diabetes.org.uk/guide-to-diabetes/managing-your-diabetes/testing (accessed Dec 2022).
- 34Giugliano D, Maiorino MI, Bellastella G, et al. Clinical inertia, reverse clinical inertia, and medication non-adherence in type 2 diabetes. J Endocrinol Invest. 2018;42:495–503. doi:10.1007/s40618-018-0951-8
- 35Giugliano D, Longo M, Caruso P, et al. Feasibility of Simplification From a Basal-Bolus Insulin Regimen to a Fixed-Ratio Formulation of Basal Insulin Plus a GLP-1RA or to Basal Insulin Plus an SGLT2 Inhibitor: BEYOND, a Randomized, Pragmatic Trial. Diabetes Care. 2021;44:1353–60. doi:10.2337/dc20-2623
- 36Lewis J. Conference over coffee: Insulin in practice, frailty and abnormal liver function tests . Diabetes on the Net. 2022.https://diabetesonthenet.com/diabetes-primary-care/welsh-pcds-2022 (accessed Dec 2022).
- 37Xultophy 100 units/ml insulin degludec + 3.6 mg/mL liraglutide solution for injection in a pre-filled pen – Summary of Product Characteristics (SmPC). Electronic Medicine Compendium. 2020.https://www.medicines.org.uk/emc/product/3469/smpc (accessed Dec 2022).
- 38Suliqua 100 units/ml + 33 micrograms/ml solution for injection in a pre-filled pen – Summary of Product Characteristics (SmPC) . Electronic Medicine Compendium. 2022.https://www.medicines.org.uk/emc/product/9871/smpc (accessed Dec 2022).
- 39Morris D. GLP-1 receptor agonists in type 2 diabetes: An underused asset? J Diabetes Nurs 2020;24.https://diabetesonthenet.com/wp-content/uploads/pdf/dotnd6c0fd23bafc13d7950c749d4760597f.pdf
- 40Khunti K, Mohan V, Jain SM, et al. Efficacy and Safety of IDegLira in Participants with Type 2 Diabetes in India Uncontrolled on Oral Antidiabetic Drugs and Basal Insulin: Data from the DUAL Clinical Trial Program. Diabetes Ther. 2017;8:673–82. doi:10.1007/s13300-017-0252-9


