Science Photo Library
After reading this article, you should be able to:
- Understand the principles of pharmacological treatment in the management of epilepsy symptoms;
- Know how the condition is monitored and how to optimise and review medication regimens;
- Understand how management strategies may differ for special patient groups.
Pharmacy teams in both primary and secondary care can support people with epilepsy, the vast majority of whom will be taking one or more anti-seizure medicines (ASMs) to manage their condition. This article is the second in a series and focuses on the management of epilepsy. Information relating to symptoms and diagnosis can be found in ‘Epilepsy: symptoms and diagnosis’.
ASMs were, until recently, referred to as ‘anti-epileptic drugs’; this term still appears in some sources. The change in terminology reflects that these drugs treat seizures, not the underlying condition. ASMs do little to relieve any of the other commonly associated symptoms of epilepsy, such as memory problems, psychological issues (e.g. depression and anxiety), and, in some cases, can exacerbate these problems[1].
Around half of all people with epilepsy will be rendered seizure-free, meaning they have not had any seizures for at least one year, with the first ASM tried; however, the probability of achieving seizure freedom reduces substantially with each subsequent ASM used[2,3]. Around 30% of patients will require polytherapy and it is not unusual for patients on multiple medications to still experience seizures[2]. More than one-third of patients’ epilepsy will remain uncontrolled, which is described as refractory to medication[4]. It is possible that ASMs will reduce the frequency or intensity of seizures in those whose epilepsy is refractory to medication, but full seizure control is difficult to achieve. Drug resistance is more likely if there is an early onset of seizures, focal seizures or multiple seizure types[4].
Patients newly diagnosed with epilepsy may still be trying to process any life changes caused by the condition and may not have had an opportunity to ask about their medication. Consultations with their neurologist may be rushed and, if they are under the care of an epilepsy nurse, much of this support may be focused on the practicalities of having epilepsy. Pharmacists can support patients with medicines management, education and counselling around medicines.
This article will cover initiation, adding on and withdrawal of ASMs, as well as describing commonly used ASMs and management considerations in special patient groups.
Starting an anti-seizure medicine
ASMs should not be started unless a diagnosis of epilepsy is confirmed. If patients do not respond to ASMs within the expected timeframe — which varies between ASMs — the diagnosis should always be reconsidered.
The commonly cited maxim for epilepsy management is that patients should be treated with monotherapy, the rationale being that this reduces the likelihood and impact of pharmacokinetic and pharmacodynamic interactions. If this does not control seizures or leads to unmanageable side effects, then the patient would be switched to a second drug as monotherapy, via a gradual titration of the first drug ‘out’ and the second drug ‘in’. The change-over regimen will be individualised to the patient, depending on whether the outgoing drug has caused intolerable side effects, and the urgency for seizure control. Titrations can be confusing for patients — pharmacists can support them by clarifying the titration details and timeline.
Evidence does not support any one approach over another for the management of seizures if the first drug does not control the condition or is poorly tolerated; in recent years, the argument has been made for rational polytherapy (i.e. the combination of ASMs with different mechanisms of action for improved efficacy)[5]. There is no agreement on whether drugs with the same mechanism of action should be combined or whether drugs with differing modes of action should be selected. It is important that the combined adverse effects are considered, particularly for drugs that impact cognition; however, it is possible that shared metabolic pathways, rather than overlapping mechanisms of action, lead to poor tolerability[1].
Awareness of common adverse effects can mean that ASMs with known side effects can be avoided in certain patient groups; for example, drugs with a higher incidence of behavioural side effects (e.g. levetiracetam) in patients with severe mental health conditions[1]. However, the side effect profiles of many of the ASMs — for both early- and late-onset effects — are similar, regardless of the drug’s mechanism of action. In the SANAD trial, a large randomised controlled trial looking at preferential therapy in 1,721 newly diagnosed patients with epilepsy, around 50% of participants reported at least one adverse effect regardless of the drug given[6,7]. The SANAD II trial demonstrated that newer ASMs, such as levetiracetam, do not necessarily offer better tolerability[8,9].
The SANAD and SANAD II trials provided information on first-line drugs that should be used in newly diagnosed epilepsy in adults and young children[6–9]. The choice is dependent on whether seizures are generalised or focal (with or without evolution to a tonic-clonic seizure). Sodium valproate has been shown in these large pragmatic randomised trials to be the most efficacious, have better tolerability and the most cost-effective drug in generalised seizures and, consequently, is the first-line treatment in men, boys and women and girls who are not of child-bearing potential[6,8]. Lamotrigine was shown to be most efficacious in controlling seizures, had the best tolerability and was cheaper in the health economic analysis of focal seizures[7,9].
ASMs are titrated slowly to minimise onset of adverse effects, with suitable licensed dosing schedules outlined in the British National Formulary (BNF); although, for some patients, titration may be undertaken even slower than indicated if they are experiencing side effects. The ‘start low, go slow’ approach enables the prescriber and patient to identify the minimally effective dose, thereby reducing side effects[10].
Adding on
Aside from the well-recognised synergism of lamotrigine and valproate, there is little evidence to guide which drug combinations should be used in which patients and when[11]. Treatment is individualised to the patient based on epilepsy syndrome, seizure type, response to previous drugs, comorbidities and other therapies, as well as patient preference, which is usually based on previous experience or what they have read about the medication[12]. The complex nature of epilepsy as a condition and considerable inter-patient variability means it is almost impossible to predict who will respond to which drug or combination of drugs, and it is not uncommon to have two patients who have seemingly similar aetiology and semiology respond differently to the same drug(s). Pharmacists should support patients by providing detailed, dated timeframes and explaining the need to follow the prescribed schedule.
Withdrawal
In some patients the decision to withdraw ASMs will be considered, usually after more than two years of seizure freedom on medication[12]. Many patients, particularly those who drive, will choose to stay on medication lifelong. ASMs should be removed slowly; rapid withdrawal can lead to rebound seizures [13]. If withdrawal is attempted, it should be done under specialist supervision, and the timing must be carefully considered for the individual patient. For example, withdrawal may be attempted in young adults before they have obtained their driving licence. A 2017 systematic review and meta-analysis of 10 studies with 1,769 patients in total showed that seizure recurrence occurred in 46% (n=812) of patients after withdrawal[13]. The longest of these recurrences was 13 years after ASM withdrawal was initiated.
Drug monitoring
There is no rationale for regular plasma drug monitoring in patients taking ASMs, although there are some instances when it is indicated (see Box 1)[14]. Individual ASMs require monitoring of other parameters (e.g. liver function tests [LFTs] for patients taking sodium valproate). These are indicated before therapy and periodically in the first six months after starting. There is no further guidance on when they should be monitored. The National Institute for Health and Care Excellence (NICE) recommends that a full blood count and other blood parameters, (e.g. urea and electrolytes, LFTs) are taken for enzyme-inducing drugs every three to five years (see ‘Useful resources‘ for list of enzyme-inducing ASMs)[12]. This should be followed for all ASMs, unless there is specific advice from the BNF or Summary of Product Characteristics that advocates for more frequent monitoring.
Box 1: Possible indications for ASM therapeutic drug monitoring
- Dose optimisation of initially prescribed treatment, including patients on phenytoin therapy and in cases of suspected toxicity with any ASM;
- Uncontrolled seizures;
- Children;
- Pregnancy;
- Older age;
- Changes in ASM formulation, including when switching from branded to generic;
- Pathological states leading to possible alterations in ASM pharmacokinetics (e.g. in hepatic or renal disease);
- Pharmacokinetic interactions (e.g. when multiple ASMs are used together).
ASMs, particularly enzyme-inducing ASMs and valproate, can negatively impact plasma vitamin D levels. Chronic use increases the risk of fracture in susceptible individuals (e.g. children and post-menopausal women)[12]. NICE currently recommends that bone metabolic tests are carried out every two to five years for adults taking enzyme-inducing ASMs and valproate[12].
Commonly used ASMs
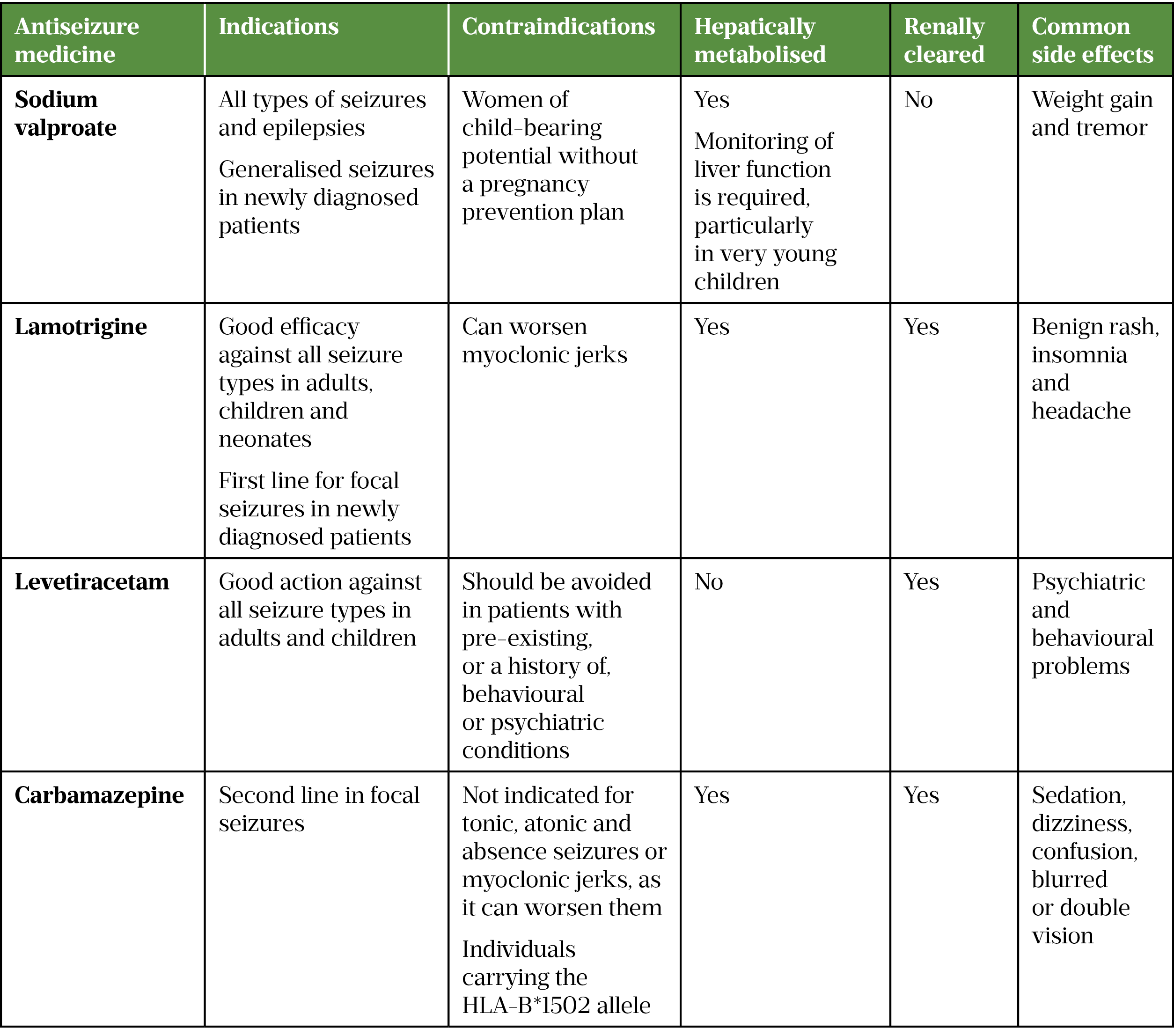
The BNF lists more than 20 drugs used to treat epilepsy; most patients will take one or more of these during their treatment journey (see Table 1)[12].
Sodium valproate
Sodium valproate is a broad spectrum ASM; however, its high teratogenicity limits its use in women of child-bearing potential, with prescribing only licensed if a Pregnancy Prevention Programme (PPP) is in place[6,8,15].
Sodium valproate is thought to interfere with metabolism of vitamin D and supplementation should be considered in patients taking the drug long-term[16]. A rare, serious side effect of long-term valproate use is pancreatitis[17]. Pharmacists should counsel patients to report symptoms of sudden abdominal pain, fever, diarrhoea and vomiting.
Sodium valproate is also an inhibitor of CYP450 enzymes and is subject to many pharmacokinetic interactions. Most notably it potentiates the effect of lamotrigine. These drugs are often prescribed together to make use of their synergistic effects whereby valproate increases the half-life of lamotrigine and decreases its clearance, meaning adjustment of initial lamotrigine dose may be required if being added to existing valproate therapy[11,17]. Sodium valproate is used clinically as a mood stabiliser and can be helpful to patients with epilepsy, who have comorbid bipolar disorder; however, a reduction in mood on withdrawal of the drug may be noted, particularly after prolonged use — patients should be warned about this and counselled to report any sudden depressed mood[17].
Lamotrigine
A broad spectrum ASM, lamotrigine has a slow-dosing titration regimen to minimise risk of serious cutaneous reactions, such as Stevens-Johnson Syndrome — a rare, potentially fatal disorder of the skin and mucous membranes that usually starts with flu-like symptoms, followed by a painful rash that spreads and blisters[18]. While lamotrigine is not metabolised via CYP450 enzymes, it is affected by enzyme-inducing ASMs and valproate, and concomitant use requires dose adjustments of lamotrigine if it is being added to the other drug. It has been suggested that its slow titration leaves people at risk of seizures for longer; however, SANAD II demonstrated that lamotrigine still outperformed other ASMs, including levetiracetam, when time to first seizure was considered[8,9].
Lamotrigine is the first-line treatment for newly diagnosed generalised seizures in women of child-bearing potential, owing to its relatively safe profile in pregnancy[6,8,19,20]. However, an interaction with oestrogen leads to enhanced glucuronidation and increased clearance, meaning that lamotrigine levels are frequently altered as the pregnancy progresses and dose adjustment is needed to avoid seizure breakthrough. Exact management of lamotrigine levels in pregnancy and when/if monitoring should be carried out is unclear. A 2018 study showed no evidence to suggest that regular monitoring of serum ASM levels in pregnancy improved seizure control or affected maternal or foetal outcomes[20]. Neurology teams tend to adopt their own approach to monitoring, depending on experience. Increased lamotrigine levels need to be corrected shortly after birth to avoid toxicity post partum.
Lamotrigine levels can be affected by the combined oral contraceptive pill and can be managed by increasing the lamotrigine dose; however, this can lead to toxicity in the pill-free period. If side effects are intolerable, patients may be advised to change their contraceptive method. The Faculty of Sexual and Reproduction Healthcare has suggested that desogestrel may increase lamotrigine levels and adverse effects[21].
Lamotrigine is used clinically to enhance mood and can be helpful to patients with epilepsy with depressive symptoms; however, a reduction in mood on withdrawal of the drug may be noted[1].
Levetiracetam
A broad spectrum ASM, despite its wide use in generalised seizures, levetiracetam is not currently licensed for monotherapy but for adjunctive treatment of myoclonic and tonic-clonic seizures[22].
Levetiracetam is extremely popular owing to its simple pharmacokinetics, lack of interactions and relatively clean safety profile[23]. Its mechanism of action differs to most ASMs — it acts by modulating synaptic neurotransmitter release by binding to the synaptic vesicle protein 2A (SV2A) in the brain; therefore, it can be useful as an adjunct therapy[23]. Levetiracetam is available as an intravenous formulation and has gained popularity as second-line treatment for status epilepticus in hospital, with patients being subsequently discharged on it[22].
SANAD II compared levetiracetam with valproate. In almost all newly diagnosed generalised seizure types valproate was shown to be superior for time to first seizure, time to treatment failure and cost-effectiveness[8]. The same trial demonstrated that levetiracetam was inferior to lamotrigine[9].
Levetiracetam is recommended for use in pregnancy; however, decreased plasma concentrations have been shown[19]. This was initially thought to primarily occur in the third trimester; however, a 2019 study suggested that maximal dose reduction arose in the first trimester[24,25]. Dose adjustments may be required to maintain seizure control and monitoring may be helpful, although, again, evidence is lacking. The precise reason for this effect is not known but it is possible that it relates to an increased volume of distribution, renal clearance and renal blood flow during pregnancy[25].
Irritability or aggression is estimated to arise in around 10–15% of patients on levetiracetam and may require withdrawal of the drug in severe cases[1]. Brivaracetam, which is the 4-n-propyl analogue of levetiracetam, is reported to have fewer psychiatric side effects owing to its more specific binding to SV2A, although it is not currently used widely in practice owing to a lack of experience in its use[25].
Carbamazepine
The SANAD I trial showed that while carbamazepine was as efficacious as lamotrigine for focal seizures, tolerability was poor in comparison[7]. Many patients still take carbamazepine but it is no longer the first-line treatment for focal seizures[26].
Carbamazepine is a potent inducer of CYP450 enzymes, meaning it has numerous significant drug interactions, including with the oestrogen and progestogen components of the contraceptive pill, which can lead to pill failure[27]. It is titrated slowly to try to minimise adverse effects, including potentially life-threatening cutaneous reactions, such as Stevens-Johnson syndrome[27].
Carbamazepine is associated with hyponatraemia, neutropenia and other blood dyscrasias[27]. Patients should be advised to report symptoms indicative of blood, liver or skin disorders to their GP as a matter of urgency. Carbamazepine is thought to interfere with metabolism of vitamin D and supplementation is recommended.
A recent Medicines and Healthcare products Regulatory Agency (MHRA) report indicates that carbamazepine is associated with an increased rate of birth abnormalities and should not be routinely recommended in pregnancy[19]. Owing to differences in bioavailability between brands, which may be clinically significant, carbamazepine is classified as an MHRA category I drug, meaning it should be prescribed by brand[28].
Carbamazepine should be avoided in patients of Han-Chinese or Tai origin who carry the HLA-B*1502 allele owing to a strong association with Stevens-Johnson syndrome[29]. Carbamazepine (and its related drugs oxcarbazepine and eslicarbazepine) should not be routinely offered to people of European or Japanese origin because of the risks of serious complications caused by association with another genetic marker HLA-A*3101[30].
Table 1 provides an overview of the four main anti-seizure medicines[7,9,17,22,31].
Benzodiazepines
Benzodiazepines have anti-seizure activity. Patients will often have a benzodiazepine added to their regimen, sometimes on a ‘when required’ basis. The two main agents for this indication are described below.
Clobazam
An oral benzodiazepine, clobazam is widely used in the management of seizures, although the evidence base on its use is lacking[32]. It is licensed as an adjunct in epilepsy. Some patients take it regularly, but it is associated with significant drowsiness that can limit its use. It is most often used intermittently as a ‘rescue’ from seizures or to ‘ward off’ seizures. For example, if a person is under stress, predicted to be going without sleep or changing between ASMs; or if focal seizures are worsening and progression to a tonic-clonic seizure is feared. Some women will use it around the time of menstruation if they experience catamenial seizures[33]. It can also be used to prevent clusters of seizures: when a person experiences several seizures throughout the day[34].
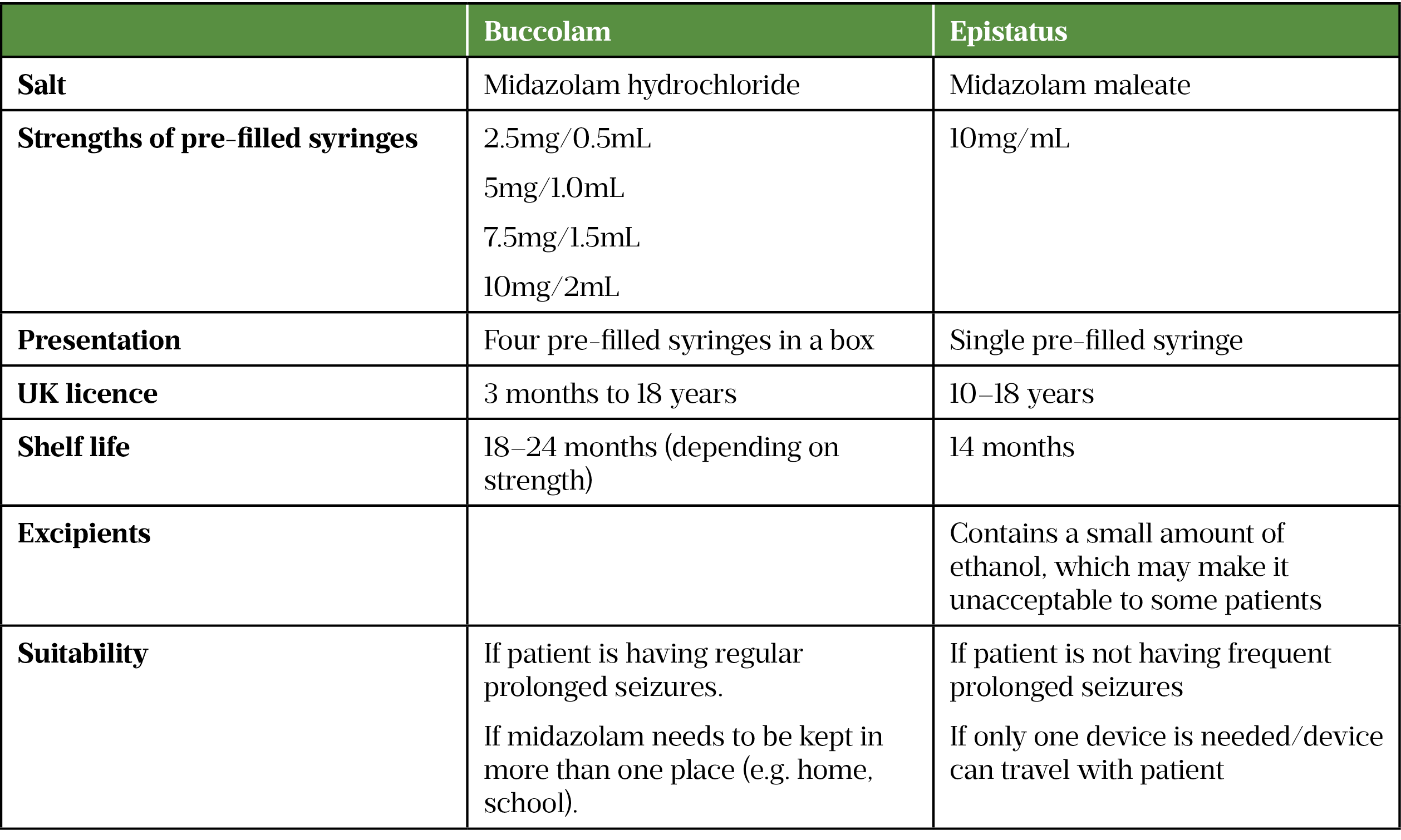
Buccal midazolam
Despite not being licensed for use in adults in the UK, buccal midazolam is recommended by NICE for prevention of status epilepticus in adults and children in the community setting[12]. It has almost completely replaced rectal diazepam, which had a very low acceptability with patients and carers when it was routinely prescribed[35]. It is prescribed for individuals who have had at least one episode of a prolonged seizure (usually one lasting five minutes). Buccal midazolam is administered via an oral syringe into the buccal cavity by a trained carer in accordance with a pre-determined, individualised protocol put in place if the person is at risk of prolonged seizures[36].
There are two different versions of midazolam available in the UK and it is important that it is prescribed by brand. The two brands are not interchangeable, and the incorrect dose has been given in some cases when the wrong product has been dispensed[37]. The product is chosen by the prescriber to suit the patient and a carer is trained in its use. If a substitution is to be made; for example, if there are availability issues, the dispensing pharmacist needs to contact the prescriber to discuss this before making a substitution. Adult patients should be dispensed the highest strength product unless otherwise stated. The dose should not be made up with multiple administrations of lower strengths. Buccal midazolam oral solutions have a relatively short shelf life, and dispensing pharmacists can help patients and carers by making a note of the expiry date and prompting when a new prescription is required. The products are not to be kept in the fridge as this renders them ineffective. In hospital, intravenous lorazepam would be used first line[38].
Table 2 provides a comparison of midazolam products licensed in the UK[39,40].
Cannabidiol
While not commonly prescribed, pharmacists are likely to receive questions about cannabidiol (CBD), owing to its high-profile introduction[41]. Cannabidiol is only licensed and NICE-approved for children and adults with the rare, serious, treatment-resistant Lennox-Gastaut and Dravet syndromes[42,43]. Unless patients had access to an early access scheme, to fulfil the NICE criteria, they need to be taking cannabidiol in conjunction with clobazam as this was shown to improve efficacy in patients with hard-to-treat seizures[42,43].
A 2018 systematic review found that 1 in every 8 people taking CBD could potentially have a 50% or greater reduction in seizures and a much smaller number (less than 1 in 150 people) could become seizure-free[44]. Side effects are common (1 in 3 people) and include drowsiness, diarrhoea, reduced appetite and fatigue[44]. Interactions with other ASMs have been reported, including significant drowsiness with clobazam and altered liver function tests with valproate. Most of the high-quality studies included in the review were carried out in paediatric patients with rare and serious forms of drug-resistant epilepsy[44]. More studies are being conducted and suggest efficacy in other epilepsy disorders, which may inform the use of cannabis-based products and cannabidiol to treat drug-resistant epilepsy in the future[45].
Adherence to ASMs
The trial-and-error approach of slow and often complex titrations, coupled with the lack of efficacy, polypharmacy, adverse effects and memory issues, can have a profound effect on adherence to ASMs[46]. Non-adherence is estimated to be around 40%, although this varies considerably depending on study type and reporting methods[46]. Non-adherence is associated with a three-fold increase in mortality and significantly increased morbidity and can prolong or worsen side effects by preventing the development of tolerance[47,48]. There is an estimated treatment gap, at least 20% more patients with epilepsy could be seizure-free if adherence to ASMs was maximised[46]. Pharmacists should support patients to improve ASM adherence where possible (see Box 2)[49,50].
Box 2: Ways in which pharmacists can support adherence in patients taking anti-seizure medicines
- Encouraging use of a seizure diary to determine the effect of medication on the number and intensity of seizures;
- Explaining possible adverse effects to look out for and what to do in the event they arise;
- Highlighting the importance of taking medication regularly to prevent breakthrough seizures;
- Advising on the best time to take a medication to fit with the patient’s routine;
- Checking patients are on the most suitable formulation (some ASMs are available as liquids, granules etc.);
- Explaining why other medication may be required (e.g. contraception for women of child-bearing potential or folic acid for women who are pregnant)
- Recommending practical methods to support adherence (multi-compartment compliance aids, alarms or reminder apps);
- Helping the patient to obtain their prescription (e.g. contacting them when it is due);
- Undertaking an emergency supply if necessary to avoid gaps in treatment;
- Helping patients obtain a consistent supply of the same medication.
Primary care pharmacists can also help where there are shared-care arrangements or where ASMs are on the local ‘red list’, indicating that consultant or specialist care is required. Patients can sometimes experience difficulties obtaining these medicines because of an error in the approval process. It is vital that people with epilepsy receive a continuous supply of their medication. Primary care pharmacists are ideally placed to navigate the complexities of local supply arrangements and prevent patients experiencing anxiety, seizure breakthrough and other effects.
The issue of generic medicines may be a cause of stress for patients who can become concerned if the appearance of their medicines changes[51]. Differences between products — for example, product name, packaging and appearance may be perceived negatively by patients and/or carers, and may lead to dissatisfaction, anxiety, confusion, dosing errors and reduced adherence. This has occurred with MHRA category 3 drugs, such as levetiracetam, where there should be no clinical difference between brands, and led to an update of the MHRA guidance in 2017, suggesting that patient concerns should be taken into consideration[28]. Discussing any changes to a patient’s medicines shape or colour prior to dispensing can help alleviate anxiety.
Being believed and listened to is fundamental for all patients, particularly those with epilepsy. Many healthcare professionals, including pharmacists, do not engage with patients with epilepsy owing to the complex nature of the care required, believing that patients are all experts in their condition and medicines, and, in some cases, fearing being responsible for a person having a seizure[52]. This results in some patients with epilepsy being denied services and care that other people would receive, contributing to the poor management of their health and their sense of isolation. This includes access to medication review when needed and referral for mental health support[53].
Epilepsy management in special patient groups
Women
There are additional considerations when prescribing for women and girls because of the possible risks associated with ASMs, all of which are teratogenic to some degree[19].
When not planning a pregnancy, women and girls of child-bearing potential require effective contraception to avoid the risk of accidental conception[54]. Enzyme-inducing drugs (see ‘Useful resources’) all have the potential to interact with hormonal contraceptives reducing contraceptive efficacy, including the implant and the patch[21]. It is recommended that women taking enzyme-inducing ASMs avoid hormonal methods of contraception; however, they are suitable for women taking a non-enzyme inducing ASM, as long as they are motivated to use the hormonal contraception correctly to maximise efficacy. If a patient has memory issues, then a user-independent method is recommended.
The MHRA PPP requires women of child-bearing potential taking sodium valproate to be using what it defines as ‘highly effective contraception’ (HEC)[15]. HEC includes the intrauterine coil, the intrauterine system and the progestogen implant, which are all user independent. More information about user-independent contraception can be found here. A woman taking valproate and an enzyme-inducing drug would be advised not to use the implant owing to the interaction with the progestogen[21]. The medroxyprogesterone injection is suitable for women taking enzyme- inducing ASMs and fulfils the requirements of the PPP. User-dependent methods, such as barrier methods and combined hormonal contraceptives (oestrogen plus progestogen) or the progestogen-only pill are not considered effective unless pill and barrier methods are combined, in which case regular pregnancy testing is recommended[15]. This may be before every prescription is issued in some cases[55].
The MHRA valproate guidance states that all women of child-bearing potential must undergo an annual risk acknowledgement consultation with a specialist that will include the completion of the Annual Risk Acknowledgment Form (ARAF)[15]. Dispensing pharmacists are required to ask if this consultation has taken place, or if the ARAF is overdue, and refer where appropriate. Practice based pharmacists can identify patients where the ARAF has not been completed and facilitate referral.
Evidence shows that babies born to women with epilepsy taking valproate during pregnancy have significantly higher rates of congenital malformations, such as cardiovascular and musculoskeletal defects, cleft palate and spina bifida, compared to the general population and to babies born to women taking other ASMs[56]. The risk of congenital malformations is around 10% in babies exposed to valproate in utero, compared to 6% and 4–5% for phenytoin and carbamazepine, respectively[19].
Subsequent evidence has shown that valproate causes neurodevelopment disorders and delay in children born to mothers who take the drug during pregnancy[57,58]. Autism and autism spectrum disorders have also been found to be higher in babies exposed to valproate in utero[59]. The MHRA guidance on valproate suggests an incidence of neurodevelopment disorders in the order of 30–40%[15].
Pregnancy
It is possible for a woman taking ASMs to have a healthy pregnancy and baby; however, the pregnancy needs to be planned. It is not usually an option to stop the ASM for the duration of the pregnancy as this leaves the woman at risk of seizures which may harm the baby[54].
Women with epilepsy who try for a baby as part of a planned pregnancy are advised to take 5mg of folic acid daily to reduce the risk of neural tube defects that can lead to major malformations such as spina bifida[54]. This is recommended for the first 12 weeks of pregnancy in all women, although the evidence on the benefits is mixed[54].
As part of planning for a pregnancy, women should be under specialist care and will ideally be transferred onto an ASM that is known to be safer in pregnancy, such as lamotrigine or levetiracetam[19]. Obtaining a pre-pregnancy plasma ASM level can be helpful when trying to maintain seizure control if plasma levels are predicted to alter during pregnancy. While there is no evidence to say how and when plasma monitoring should be carried out during pregnancy, levels are usually checked at each trimester. After pregnancy, it is important that the epilepsy specialist arranges for the dose to be reduced as quickly as possible to ensure pre-pregnancy plasma ranges, particularly if the woman plans to breastfeed. The UK has a pregnancy register that women with epilepsy are invited to join to inform future practice[60].
The risk of SUDEP is increased in pregnant women with epilepsy and for 12 months after birth[61]. Possible reasons for this are adherence and sleep deprivation.
Pharmacists should counsel women with epilepsy who they think might be considering pregnancy on the need for planned pregnancy, specialist review and regular monitoring during pregnancy.
Breastfeeding
Breastfeeding is usually safe for women who are taking ASMs and may confer benefits in terms of improved cognitive outcomes in children of women with epilepsy who were taking ASMs[62–64]. A report from the American Academy of Neurology and the American Epilepsy Society suggested that levetiracetam transfers into breastmilk at a clinically significant level[65]. Precise transfer levels that affect neonatal and childhood outcomes are not known, but the midwife/health visitor should advise on the timing of feeds to avoid higher drug concentrations in the infant[54]. If the baby appears drowsy, develops a rash or is failing to thrive, the woman should report this to her midwife at once.
Parents should receive advice on caring for their baby to reduce seizure-associated risks. This includes avoiding bathing the baby alone and changing the baby on the floor[54].
Menopause and bone health
Some women with epilepsy may experience a change in seizure control around the time of the menopause, whether this is an improvement or a worsening[66]. The oestrogen in hormone replacement therapy may interact with ASMs as outlined above and can lead to worsening of seizures[67]. Enzyme-inducing ASMs and valproate are thought to affect the metabolism of vitamin D and supplementation of 10–20 micrograms for adults is recommended for people taking these medicines long-term[68]. Supplementation is particularly important for women at additional risk of osteoporosis (e.g. family history, early menopause, overactive thyroid)[68].
Patients with intellectual disability
One systematic review estimated that the prevalence of epilepsy in people with an intellectual disability (ID) is as high as 22%[69]. It can be challenging to determine the type of seizures that are being experienced and the frequency as they can be confused with other behaviours associated with the person’s condition (e.g. jerking, twitching or shouting). Management of epilepsy can be difficult in this patient group owing to physical and psychiatric comorbidity, complex behaviours, problems performing investigations and communication difficulties. Generally, patients with ID are excluded from drug trials so evidence on which medications are most effective is lacking.
In 2017, the Royal College of Psychiatrists published a report to offer guidance on individual agents and some general prescribing principles for ASMs in this group[70]. It stresses the need to take a person-centred approach to prescribing ASMs and other medicines. Prescribers are advised to be mindful of the risks of using ASMs that affect behaviour or cognition (e.g. levetiracetam). The report highlights the role of the pharmacist in the management of complex medication issues in patients with ID. Pharmacists can aid the optimisation of ASMs in this patient group and avoid the over-use of medication[71,72]. The Step Together document (see ‘Useful resources‘) proposes models for integrating care.
Children
Epilepsy in young children is usually managed by the paediatric neurology team; it is a very specialised area and is beyond the scope of this article.
Non-pharmacological management
There is little evidence available for the non-pharmacological management of epilepsy, although many patients will try complementary and alternative therapies. Case reports have shown that some of these therapies have the potential to be pro-convulsant (e.g. ginkgo bilboa, eucalyptus and star anise) and pharmacists may be able to advise on the appropriate use of these treatments[73].
Keeping a seizure diary can help identify triggers and necessary lifestyle interventions; for example, keeping alcohol intake to a minimum and implementing sleep hygiene where appropriate. For some patients, counselling and cognitive behavioural therapy has proved effective in helping them feel in control of their seizures and in managing comorbid depression and anxiety[74].
For patients with refractory focal epilepsy, surgery may be considered. This can lead to seizure freedom for some patients, although it is generally under-utilised owing to the associated risks of surgery on the brain and because it is not suitable for many patients owing to other comorbidities[75]. Patient suitability needs to be carefully assessed with appropriate investigations. These pre-surgical investigations, including scans such as functional MRI and neuropsychological assessment, will ensure that the location for epileptogenesis can be identified and that resection will not lead to significant issues afterwards, such as worsened memory or speech. Vagus nerve stimulation is where a device which stimulates the left vagus nerve is inserted into the chest to reduce abnormal electrical activity leading to seizures. Deep brain stimulation involves the implantation of stimulating electrodes directly into specific areas of the brain and may be considered when there is not a single lesion or seizure focus suitable for resection. These may reduce seizure frequency and severity, but rarely bring seizure freedom[76].
In children, the ketogenic diet has been shown to be effective at reducing seizure frequency, although the evidence is not high quality and the precise effect it provides is uncertain[77]. Its efficacy in adults has not been demonstrated[77].
Wearable technology, alarms, monitors and other seizure detectors have not been shown in trials to be effective in reducing seizure frequency, but many patients and carers do invest in them.
Patient monitoring
There is no evidence to support a certain frequency of monitoring in patients with epilepsy. Current NICE guidelines suggest annual review with a patient’s GP or epilepsy specialist, but this has become less likely, particularly with the removal of annual review from the Quality and Outcomes Framework for GPs[12]. Perceived wisdom would be that patients with additional needs (be that seizures or other epilepsy-related conditions) need more regular review. Given the poor outcomes owing to comorbidities in patients with epilepsy, regular disease management for other conditions should also be prioritised.
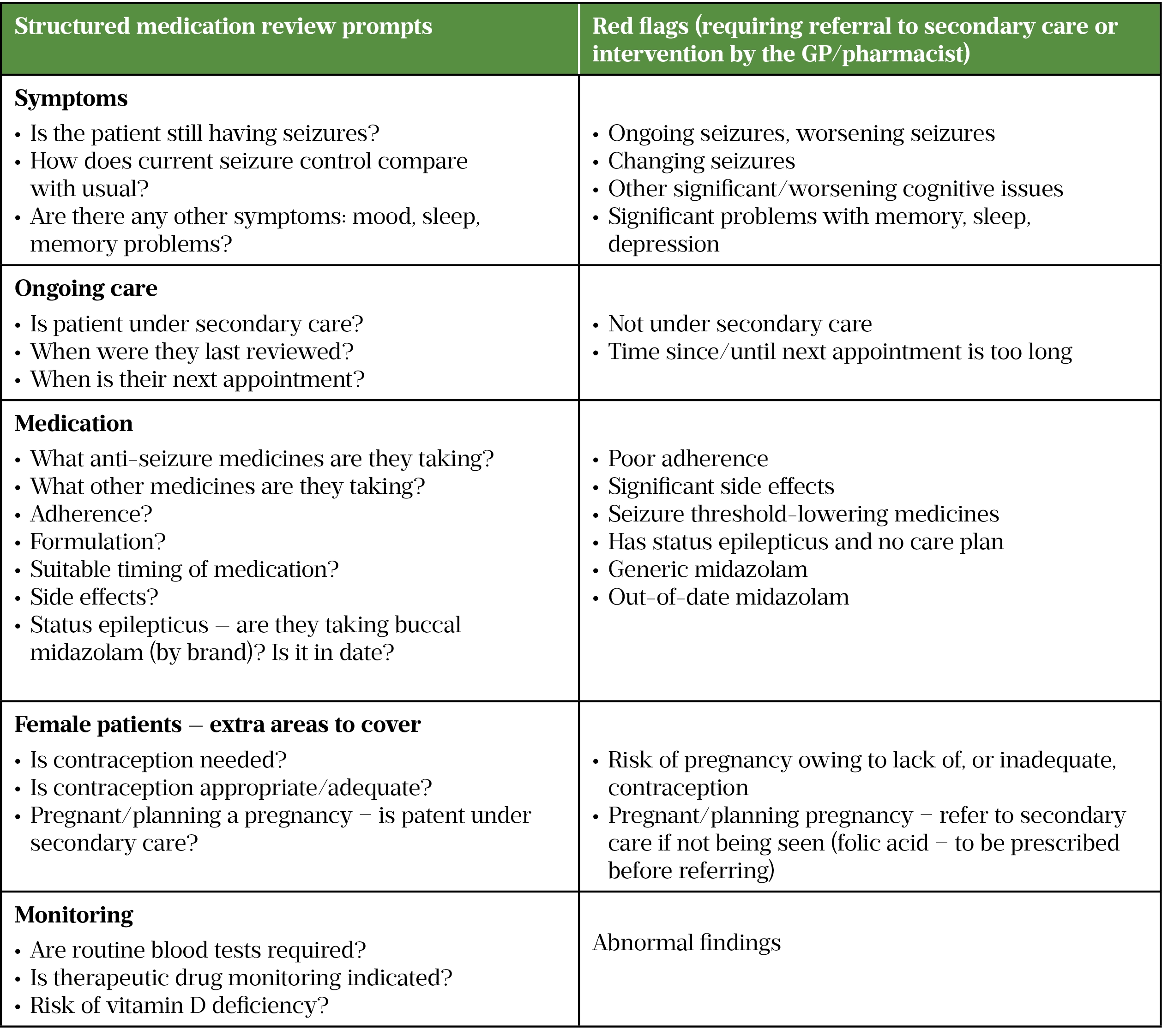
Structured medication review
For pharmacists working in primary care, a structured medication review offers patients the opportunity to ask questions and raise any concerns they may have. There is no evidence to show that annual reviews for all patients with a diagnosis of epilepsy are necessary; however, patients with uncontrolled epilepsy, worsening seizures or other symptoms (e.g. issues with sleep or depression) should be seen more regularly, depending on clinical need. Suggestions for what could be included in a structured medication review are shown in Table 3.
From 1 September 2021, the New Medicine Service (NMS) has included patients with epilepsy[78]. This service should enable pharmacists to identify any side effects that a patient might be experiencing and help improve patient adherence.
The Discharge Medicines Service enables hospital pharmacists to refer patients to community colleagues for support[79]. Community pharmacists can then undertake a patient-centred discussion to ensure the patient understands their medicine regimen, including any changes made while being treated by the hospital. This could be particularly beneficial to patients with epilepsy, who have been started on medication because of a first seizure or after a prolonged seizure, where they may be confused and struggle to comprehend the information they receive at the time.
Community pharmacists can support patients who are newly started on ASMs; for example, liaising with hospital colleagues if the patient has been referred through the Discharge Medicines Service. All pharmacists should advise on possible interactions and other medicines to avoid.
Conclusion
Regardless of the setting, pharmacists can support people with epilepsy with their medication, encouraging adherence and acting as a valuable resource for this vulnerable, high-risk group of patients[49,80]. Even if pharmacists are not involved with the management of the patient’s epilepsy directly, advising on the treatment of other comorbidities in this group of patients utilises the skills of pharmacists and can help to prevent premature death.
Useful resources
- The Epilepsy Society (and its guidance specifically for pharmacists);
- Epilepsy Action (specifically its guidance on contraception with anti-seizure medicines);
- SUDEP Action;
- DVLA – ‘Epilepsy and driving‘;
- Step Together – ‘Integrating care for people with a learning disability and epilepsy‘;
- The National Institute for Health and Care Excellence’s list of enzyme-inducing antiepileptic drugs.
- 1Weintraub D, Buchsbaum R, Resor SR Jr, et al. Psychiatric and behavioral side effects of the newer antiepileptic drugs in adults with epilepsy. Epilepsy & Behavior. 2007;10:105–10. doi:10.1016/j.yebeh.2006.08.008
- 2Kwan P, Brodie MJ. Early Identification of Refractory Epilepsy. N Engl J Med. 2000;342:314–9. doi:10.1056/nejm200002033420503
- 3Chen Z, Brodie MJ, Liew D, et al. Treatment Outcomes in Patients With Newly Diagnosed Epilepsy Treated With Established and New Antiepileptic Drugs. JAMA Neurol. 2018;75:279. doi:10.1001/jamaneurol.2017.3949
- 4Sultana B, Panzini M-A, Veilleux Carpentier A, et al. Incidence and Prevalence of Drug-Resistant Epilepsy. Neurology. 2021;96:805–17. doi:10.1212/wnl.0000000000011839
- 5Lee JW, Dworetzky B. Rational Polytherapy with Antiepileptic Drugs. Pharmaceuticals. 2010;3:2362–79. doi:10.3390/ph3082362
- 6Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trial. The Lancet. 2007;369:1016–26. doi:10.1016/s0140-6736(07)60461-9
- 7Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. The Lancet. 2007;369:1000–15. doi:10.1016/s0140-6736(07)60460-7
- 8Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalised and unclassifiable epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomised controlled trial. The Lancet. 2021;397:1375–86. doi:10.1016/s0140-6736(21)00246-4
- 9Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of levetiracetam, zonisamide, or lamotrigine for newly diagnosed focal epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomised controlled trial. The Lancet. 2021;397:1363–74. doi:10.1016/s0140-6736(21)00247-6
- 10Ferrendelli JA. Concerns with Antiepileptic Drug Initiation: Safety, Tolerability, and Efficacy. Epilepsia. 2001;42:28–30. doi:10.1046/j.1528-1157.2001.0420s4028.x
- 11Brodie MJ, Yuen AWC. Lamotrigine substitution study: evidence for synergism with sodium valproate? Epilepsy Research. 1997;26:423–32. doi:10.1016/s0920-1211(96)01007-8
- 12Epilepsies: diagnosis and management: Clinical guideline [CG137]. National Institute for Health and Care Excellence. 2012.https://www.nice.org.uk/guidance/cg137 (accessed May 2022).
- 13Lamberink HJ, Otte WM, Geerts AT, et al. Individualised prediction model of seizure recurrence and long-term outcomes after withdrawal of antiepileptic drugs in seizure-free patients: a systematic review and individual participant data meta-analysis. The Lancet Neurology. 2017;16:523–31. doi:10.1016/s1474-4422(17)30114-x
- 14Patsalos PN, Spencer EP, Berry DJ. Therapeutic Drug Monitoring of Antiepileptic Drugs in Epilepsy: A 2018 Update. Therapeutic Drug Monitoring. 2018;40:526–48. doi:10.1097/ftd.0000000000000546
- 15Guidance: Valproate use by women and girls. Medicines and Healthcare products Regulatory Agency. 2018.https://www.gov.uk/guidance/valproate-use-by-women-and-girls (accessed May 2022).
- 16Siniscalchi A, Murphy S, Cione E, et al. Antiepileptic Drugs and Bone Health: Current Concepts. Psychopharmacol Bull 2020;50:36–44.https://www.ncbi.nlm.nih.gov/pubmed/32508365
- 17Sodium valproate. In: BNF 80. London: : Pharmaceutical Press 2020. 1768.
- 18Lamotrigine: Adverse effects. In: BNF 80. London: : Pharmaceutical Press 2020. 1768.
- 19Antiepileptic drugs: review of safety of use during pregnancy. MHRA UK Public Assessment Report January 2021. Medicines and Healthcare products Regulatory Agency. 2021.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/950066/AED-PAR-PDF-FINAL-Jan21.pdf (accessed May 2022).
- 20Thangaratinam S, Marlin N, Newton S, et al. AntiEpileptic drug Monitoring in PREgnancy (EMPiRE): a double-blind randomised trial on effectiveness and acceptability of monitoring strategies. Health Technol Assess. 2018;22:1–152. doi:10.3310/hta22230
- 21FSRH CEU Guidance: Drug Interactions with Hormonal Contraception (January 2017, last reviewed 2019). Faculty of Sexual and Reproductive Health. 2017.https://www.fsrh.org/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/ (accessed May 2022).
- 22Levetiracetam: indication and dose . In: BNF 80. London: : Pharmaceutical Press 2020. 1768.
- 23Patsalos PN. Pharmacokinetic profile of levetiracetam. Pharmacology & Therapeutics. 2000;85:77–85. doi:10.1016/s0163-7258(99)00052-2
- 24Westin AA, Reimers A, Helde G, et al. Serum concentration/dose ratio of levetiracetam before, during and after pregnancy. Seizure. 2008;17:192–8. doi:10.1016/j.seizure.2007.11.027
- 25Berlin M, Barchel D, Gandelman-Marton R, et al. Therapeutic levetiracetam monitoring during pregnancy: “mind the gap”. Therapeutic Advances in Chronic Disease. 2019;10:204062231985165. doi:10.1177/2040622319851652
- 26Epilepsies in children, young people and adults: NICE guideline [NG217]. National Institute for Health and Care Excellence. 2022.https://www.nice.org.uk/guidance/NG217/history (accessed May 2022).
- 27Carbamazepine . In: BNF 80. London: : Pharmaceutical Press 2020. 1768.
- 28Antiepileptic drugs: updated advice on switching between different manufacturers’ products. Medicines and Healthcare products Regulatory Agency. 2017.https://www.gov.uk/drug-safety-update/antiepileptic-drugs-updated-advice-on-switching-between-different-manufacturers-products (accessed May 2022).
- 29Ferrell P, McLeod H. Carbamazepine, HLA-B*1502 and risk of Stevens-Johnson syndrome and toxic epidermal necrolysis: US FDA recommendations. Pharmacogenomics 2008;9:1543–6. doi:10.2217/14622416.9.10.1543
- 30Carbamazepine, oxcarbazepine and eslicarbazepine: potential risk of serious skin reactions. Medicines and Healthcare products Regulatory Agency. 2014.https://www.gov.uk/drug-safety-update/carbamazepine-oxcarbazepine-and-eslicarbazepine-potential-risk-of-serious-skin-reactions (accessed May 2022).
- 31Striano P, Belcastro V. Treatment of myoclonic seizures. Expert Rev Neurother 2012;12:1411–7; quiz 1418. doi:10.1586/ern.12.90
- 32Pernea M, Sutcliffe A. Clobazam and Its Use in Epilepsy. Pediatr Rep 2016;8:6516. doi:10.4081/pr.2016.6516
- 33Verrotti Alberto A, D’Egidio, Agostinelli, et al. Diagnosis and management of catamenial seizures: a review. IJWH. 2012;:535. doi:10.2147/ijwh.s28872
- 34Jafarpour S, Hirsch LJ, Gaínza-Lein M, et al. Seizure cluster: Definition, prevalence, consequences, and management. Seizure. 2019;68:9–15. doi:10.1016/j.seizure.2018.05.013
- 35Khan A, Baheerathan A, Setty G, et al. G223(P) A Community-Based Study on the Acceptability, Efficacy and Safety of Buccal Midzolam in Children with Epilepsy. Archives of Disease in Childhood. 2013;98:A99–100. doi:10.1136/archdischild-2013-304107.235
- 36Best practice guidelines for training professional carers in the administration of Buccal (Oromucosal) Midazolam for the treatment of prolonged and / or clusters of epileptic seizures in the community. Epilepsy Nurses Association. 2019.https://ilaebritish.org.uk/content/uploads/2019/06/ESNA-Midazolam-Guidelines.pdf (accessed May 2022).
- 37Medication Safety Alert: Prescribing of Buccal Midazolam. NHS Hull and East Riding Prescribing Committee. 2012.https://www.hey.nhs.uk/wp/wp-content/uploads/2016/03/safetyAlertBuccalMidazolam.pdf (accessed May 2022).
- 38Betjemann JP, Lowenstein DH. Status epilepticus in adults. The Lancet Neurology. 2015;14:615–24. doi:10.1016/s1474-4422(15)00042-3
- 39BUCCOLAM 10 mg oromucosal solution. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/7460/smpc (accessed May 2022).
- 40Epistatus 10 mg Oromucosal Solution. Electronic Medicines Compendium. https://www.medicines.org.uk/emc/product/2679 (accessed May 2022).
- 41Medical Cannabis (Access) Bill Volume 705: debated on Friday 10 December 2021. UK Parliament. 2021.https://hansard.parliament.uk/commons/2021-12-10/debates/3412378D-471C-4ED8-A315-73CA97C9A6DB/MedicalCannabis(Access)Bill (accessed May 2022).
- 42Cannabidiol with clobazam for treating seizures associated with Lennox–Gastaut syndrome: Technology appraisal guidance [TA615]. National Institute for Health and Care Excellence. 2019.https://www.nice.org.uk/guidance/ta615 (accessed May 2022).
- 43Cannabidiol with clobazam for treating seizures associated with Dravet syndrome: Technology appraisal guidance [TA614]. National Institute for Health and Care Excellence. 2019.https://www.nice.org.uk/guidance/ta614 (accessed May 2022).
- 44Stockings E, Zagic D, Campbell G, et al. Evidence for cannabis and cannabinoids for epilepsy: a systematic review of controlled and observational evidence. J Neurol Neurosurg Psychiatry. 2018;89:741–53. doi:10.1136/jnnp-2017-317168
- 45Lattanzi S, Trinka E, Striano P, et al. Highly Purified Cannabidiol for Epilepsy Treatment: A Systematic Review of Epileptic Conditions Beyond Dravet Syndrome and Lennox–Gastaut Syndrome. CNS Drugs. 2021;35:265–81. doi:10.1007/s40263-021-00807-y
- 46Malek N, Heath CA, Greene J. A review of medication adherence in people with epilepsy. Acta Neurol Scand. 2016;135:507–15. doi:10.1111/ane.12703
- 47Faught E, Duh MS, Weiner JR, et al. Nonadherence to antiepileptic drugs and increased mortality: Findings from the RANSOM Study. Neurology. 2008;71:1572–8. doi:10.1212/01.wnl.0000319693.10338.b9
- 48Loscher W, Schmidt D. Experimental and Clinical Evidence for Loss of Effect (Tolerance) during Prolonged Treatment with Antiepileptic Drugs. Epilepsia. 2006;47:1253–84. doi:10.1111/j.1528-1167.2006.00607.x
- 49Fogg A, Staufenberg EF, Small I, et al. An exploratory study of primary care pharmacist-led epilepsy consultations. International Journal of Pharmacy Practice. 2012;20:294–302. doi:10.1111/j.2042-7174.2012.00207.x
- 50Koshy S. Role of pharmacists in the management of patients with epilepsy. International Journal of Pharmacy Practice. 2011;20:65–8. doi:10.1111/j.2042-7174.2011.00156.x
- 51Berg MJ, Gross RA, Haskins LS, et al. Generic substitution in the treatment of epilepsy: Patient and physician perceptions. Epilepsy & Behavior. 2008;13:693–9. doi:10.1016/j.yebeh.2008.06.001
- 52Schon F. Is clinical neurology really so difficult? Journal of Neurology, Neurosurgery & Psychiatry. 2002;72:557–9. doi:10.1136/jnnp.72.5.557
- 53RightCare: Epilepsy Toolkit Optimising a system for people living with epilepsy. NHS England. 2020.https://www.england.nhs.uk/rightcare/wp-content/uploads/sites/40/2020/03/rightcare-epilepsy-toolkit-v2.pdf (accessed May 2022).
- 54Epilepsy in Pregnancy (Green-top Guideline No. 68). Royal College of Obstetricians and Gynaecologists. 2016.https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/epilepsy-in-pregnancy-green-top-guideline-no-68/ (accessed May 2022).
- 55Pregnancy testing and contraception for pregnancy prevention during treatment with medicines of teratogenic potential. Medicines and Healthcare products Regulatory Agency. 2019.https://assets.publishing.service.gov.uk/media/5c936a4840f0b633f5bfd895/pregnancy_testing_and_contraception_table_for_medicines_with_teratogenic_potential_final.pdf (accessed May 2022).
- 56Weston J, Bromley R, Jackson CF, et al. Monotherapy treatment of epilepsy in pregnancy: congenital malformation outcomes in the child. Cochrane Database of Systematic Reviews. 2016. doi:10.1002/14651858.cd010224.pub2
- 57Meador KJ, Baker GA, Browning N, et al. Cognitive Function at 3 Years of Age after Fetal Exposure to Antiepileptic Drugs. N Engl J Med. 2009;360:1597–605. doi:10.1056/nejmoa0803531
- 58Meador KJ, Baker GA, Browning N, et al. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. The Lancet Neurology. 2013;12:244–52. doi:10.1016/s1474-4422(12)70323-x
- 59Christensen J, Grønborg T, Sørensen M, et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 2013;309:1696–703. doi:10.1001/jama.2013.2270
- 60UK Epilepsy and Pregnancy Register. UK Epilepsy and Pregnancy Register. http://www.epilepsyandpregnancy.co.uk/ (accessed May 2022).
- 61Knight M, Bunch K, Tuffnell D, et al. Saving Lives, Improving Mothers’ Care: Lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2016-18. National Perinatal Epidemiology Unit, University of Oxford . 2020.https://www.npeu.ox.ac.uk/assets/downloads/mbrrace-uk/reports/maternal-report-2020/MBRRACE-UK_Maternal_Report_Dec_2020_v10_ONLINE_VERSION_1404.pdf (accessed May 2022).
- 62Noe K. Further Evidence Breastfeeding by Women With Epilepsy Is Safe: Are Mothers Getting the Message? Epilepsy Curr. 2020;20:141–3. doi:10.1177/1535759720917997
- 63Meador KJ, Baker GA, Browning N, et al. Breastfeeding in Children of Women Taking Antiepileptic Drugs. JAMA Pediatr. 2014;168:729. doi:10.1001/jamapediatrics.2014.118
- 64Veiby G, Bjørk M, Engelsen BA, et al. Epilepsy and recommendations for breastfeeding. Seizure. 2015;28:57–65. doi:10.1016/j.seizure.2015.02.013
- 65Harden CL, Pennell PB, Koppel BS, et al. Management issues for women with epilepsy-Focus on pregnancy (an evidence-based review): III. Vitamin K, folic acid, blood levels, and breast-feeding. Epilepsia. 2009;50:1247–55. doi:10.1111/j.1528-1167.2009.02130.x
- 66Kennedy JD, Chen MJ. Women & Epilepsy. Practical Neurology. 2019.https://practicalneurology.com/articles/2019-oct/women-epilepsy (accessed May 2022).
- 67Harden C, Herzog A, Nikolov B, et al. Hormone replacement therapy in women with epilepsy: a randomized, double-blind, placebo-controlled study. Epilepsia 2006;47:1447–51. doi:10.1111/j.1528-1167.2006.00507.x
- 68Antiepileptics: adverse effects on bone. Medicines and Healthcare products Regulatory Agency. 2014.https://www.gov.uk/drug-safety-update/antiepileptics-adverse-effects-on-bone (accessed May 2022).
- 69Robertson J, Hatton C, Emerson E, et al. Prevalence of epilepsy among people with intellectual disabilities: A systematic review. Seizure. 2015;29:46–62. doi:10.1016/j.seizure.2015.03.016
- 70Prescribing anti-epileptic drugs for people with epilepsy and intellectual disability. Royal College of Psychiatrists. 2017.https://www.rcpsych.ac.uk/docs/default-source/improving-care/better-mh-policy/college-reports/college-report-cr206.pdf?sfvrsn=4db7a660_2 (accessed May 2022).
- 71O’Dwyer M, Watkins L, McCallion P, et al. Optimising medicines use in older adults with intellectual disability who have epilepsy: challenges and perspectives. Therapeutic Advances in Drug Safety. 2021;12:204209862110251. doi:10.1177/20420986211025157
- 72Branford D, Gerrard D, Saleem N, et al. Stopping over-medication of people with intellectual disability, Autism or both (STOMP) in England part 1 – history and background of STOMP. AMHID. 2019;13:31–40. doi:10.1108/amhid-02-2018-0004
- 73Samuels N, Finkelstein Y, Singer S, et al. Herbal medicine and epilepsy: proconvulsive effects and interactions with antiepileptic drugs. Epilepsia 2008;49:373–80. doi:10.1111/j.1528-1167.2007.01379.x
- 74Leeman-Markowski B, Schachter S. Cognitive and Behavioral Interventions in Epilepsy. Curr Neurol Neurosci Rep 2017;17:42. doi:10.1007/s11910-017-0752-z
- 75Vakharia V, Duncan J, Witt J, et al. Getting the best outcomes from epilepsy surgery. Ann Neurol 2018;83:676–90. doi:10.1002/ana.25205
- 76DeGiorgio C, Schachter S, Handforth A, et al. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia 2000;41:1195–200. doi:10.1111/j.1528-1157.2000.tb00325.x
- 77Martin-McGill KJ, Bresnahan R, Levy RG, et al. Ketogenic diets for drug-resistant epilepsy. Cochrane Database of Systematic Reviews. 2020;2020. doi:10.1002/14651858.cd001903.pub5
- 78New Medicine Service (NMS). Pharmaceutical Services Negotiating Committee. https://psnc.org.uk/services-commissioning/advanced-services/nms/ (accessed May 2022).
- 79Discharge Medicines Service. Pharmaceutical Services Negotiating Committee. https://psnc.org.uk/services-commissioning/essential-services/discharge-medicines-service/ (accessed May 2022).
- 80Marawar R, Faraj M, Lucas K, et al. Implementation of an older adult epilepsy clinic utilizing pharmacist services. J Am Pharm Assoc (2003) 2021;61:e93–8. doi:10.1016/j.japh.2021.07.003


